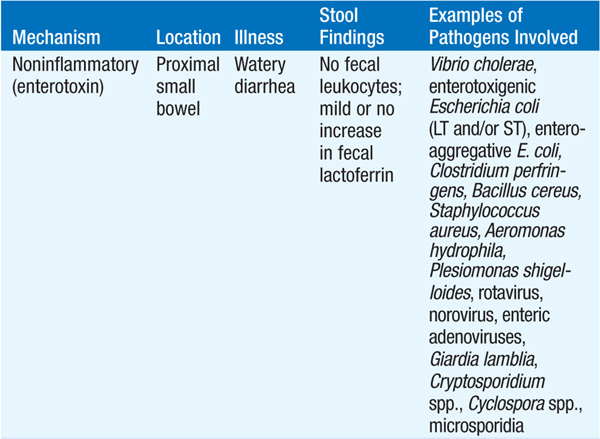
Acute diarrheal disease, which has an incidence of ~4.6 billion cases worldwide per year, is the second most common infectious cause of death worldwide (after lower respiratory tract infection). The wide range of clinical manifestations is matched by the wide variety of infectious agents involved (Table 91-1). An approach to pts with infectious diarrhea is presented in Fig. 91-1.
TABLE 91-1 GASTROINTESTINAL PATHOGENS CAUSING ACUTE DIARRHEA

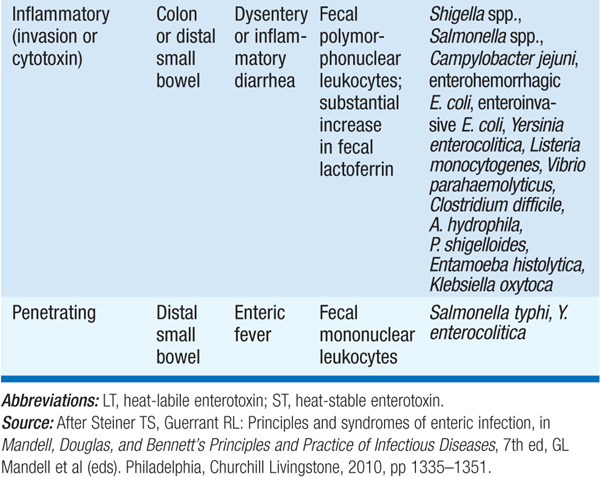
FIGURE 91-1 Clinical algorithm for the approach to pts with community-acquired infectious diarrhea or bacterial food poisoning. Key to superscripts: 1. Diarrhea lasting >2 weeks is generally defined as chronic; in such cases, many of the causes of acute diarrhea are much less likely, and a new spectrum of causes needs to be considered. 2. Fever often implies invasive disease, although fever and diarrhea may also result from infection outside the GI tract, as in malaria. 3. Stools that contain blood or mucus indicate ulceration of the large bowel. Bloody stools without fecal leukocytes should alert the laboratory to the possibility of infection with Shiga toxin–producing enterohemorrhagic Escherichia coli. Bulky white stools suggest a small-intestinal process that is causing malabsorption. Profuse “rice-water” stools suggest cholera or a similar toxigenic process. 4. Frequent stools over a given period can provide the first warning of impending dehydration. 5. Abdominal pain may be most severe in inflammatory processes like those due to Shigella, Campylobacter, and necrotizing toxins. Painful abdominal muscle cramps, caused by electrolyte loss, can develop in severe cases of cholera. Bloating is common in giardiasis. An appendicitis-like syndrome should prompt a culture for Yersinia enterocolitica with cold enrichment. 6. Tenesmus (painful rectal spasms with a strong urge to defecate but little passage of stool) may be a feature of cases with proctitis, as in shigellosis or amebiasis. 7. Vomiting implies an acute infection (e.g., a toxin-mediated illness or food poisoning) but can also be prominent in a variety of systemic illnesses (e.g., malaria) and in intestinal obstruction. 8. Asking pts whether anyone else they know is sick is a more efficient means of identifying a common source than is constructing a list of recently eaten foods. If a common source seems likely, specific foods can be investigated. See text for a discussion of bacterial food poisoning. 9. Current antibiotic therapy or a recent history of treatment suggests Clostridium difficile diarrhea. Stop antibiotic treatment if possible and consider tests for C. difficile toxins. Antibiotic use may increase the risk of other infections, such as salmonellosis. 10. See Chap. 214 for a discussion of traveler’s diarrhea. [After Steiner TS, Guerrant RL: Principles and syndromes of enteric infection, in Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 7th ed, GL Mandell et al (eds). Philadelphia, Churchill Livingstone, 2010, pp 1335–1351; RL Guerrant, DA Bobak: N Engl J Med 325:327, 1991; with permission.]
See Chap. 214 for details.
If there is evidence of a common-source outbreak, questions concerning the ingestion of specific foods and the timing of the diarrhea after a meal can provide clues to the bacterial cause of the illness.
• Staphylococcus aureus: Enterotoxin is elaborated in food left at room temperature (e.g., at picnics).
– The incubation period is 1–6 h. Disease lasts <12 h and consists of diarrhea, nausea, vomiting, and abdominal cramping, usually without fever.
– Most cases are due to contamination from infected human carriers.
• Bacillus cereus: Either an emetic or a diarrheal form of food poisoning can occur.
– The emetic form presents like S. aureus food poisoning, is due to a staphylococcal type of enterotoxin, has an incubation period of 1–6 h, and is associated with contaminated fried rice.
– The diarrheal form has an incubation period of 8–16 h, is caused by an enterotoxin resembling Escherichia coli heat-labile toxin (LT), and presents with diarrhea and abdominal cramps without vomiting.
• Clostridium perfringens: Ingestion of heat-resistant spores in undercooked meat, poultry, or legumes leads to toxin production in the intestinal tract. The incubation period is 8–14 h, after which pts develop ≤24 h of diarrhea and abdominal cramps, without vomiting or fever.
Cholera is caused by Vibrio cholerae serogroups O1 (classic and El Tor biotypes) and O139—highly motile, facultatively anaerobic, curved gram-negative rods. The natural habitat of V. cholerae is coastal salt water and brackish estuaries. Toxin production causes disease manifestations.
Currently, >90% of cases reported to the World Health Organization (WHO) are from Africa; however, most cases go unreported or do not have a specific bacterial etiology identified.
• It is estimated that there are >3 million cases annually, with >100,000 deaths.
• Spread takes place by fecal contamination of water and food sources. Infection requires ingestion of a relatively large inoculum (compared with that required for other pathogens) of >105 organisms.
After an incubation period of 24–48 h, pts develop painless watery diarrhea and vomiting that can cause profound, rapidly progressive dehydration and death within hours.
• Volume loss can be >250 mL/kg in the first day.
• Stool has a characteristic “rice-water” appearance: gray cloudy fluid with flecks of mucus; no blood; and a fishy, inoffensive odor.
Stool cultures on selective medium [e.g., thiosulfate–citrate–bile salts–sucrose (TCBS) agar] can isolate the organism. A point-of-care antigen-detection assay is available for field use.
TREATMENT Cholera
Rapid fluid replacement is critical, preferably with the WHO’s reduced-osmolarity oral rehydration solution (ORS), which contains (per liter of water) Na+, 75 mmol; K+, 20 mmol; Cl–, 65 mmol; citrate, 10 mmol; and glucose, 75 mmol.
• If available, rice-based ORS is considered superior to standard ORS for cholera.
• If ORS is not available, a substitute can be made by adding 0.5 teaspoon of table salt (NaCl; 3.5 g) and 4 tablespoons of table sugar (glucose; 40 g) to 1 L of safe water.
• Severely dehydrated pts should be managed initially with IV hydration (preferably with Ringer’s lactate), with the total fluid deficit replaced in the first 3–6 h (half within the first hour).
• A single dose of an effective antibiotic diminishes the duration and volume of stool: doxycycline (300 mg), ciprofloxacin (30 mg/kg, not to exceed 1 g), or azithromycin (1 g).
These infections are linked to ingestion of contaminated seawater or undercooked seafood. After an incubation period of 4 h to 4 days, watery diarrhea, abdominal cramps, nausea, vomiting, and occasionally fever and chills develop. The disease lasts <7 days. Dysentery is a less common presentation. Pts with comorbid disease (e.g., liver disease) sometimes have extraintestinal infections that require antibiotic treatment.
These single-stranded RNA viruses are common causes of traveler’s diarrhea and of viral gastroenteritis in pts of all ages as well as of epidemics worldwide, with a higher prevalence in cold-weather months. In the United States, >90% of outbreaks of nonbacterial gastroenteritis are caused by noroviruses. Very small inocula are required for infection. Thus, although the fecal-oral route is the primary mode of transmission, aerosolization, fomite contact, and person-to-person contact can also result in infection.
After a 24-h incubation period (range, 12–72 h), pts experience the sudden onset of nausea, vomiting, diarrhea, and/or abdominal cramps with constitutional symptoms (e.g., fever, headache, chills). Stools are loose, watery, and without blood, mucus, or leukocytes. Disease lasts 12–60 h.
PCR assays and enzyme immunoassays (EIAs) have been developed to detect these viruses in stool and other body fluids, but these techniques are still largely relegated to research and outbreak settings.
TREATMENT Infections with Noroviruses and Related Human Caliciviruses
Only supportive measures are required.
Rotavirus is a segmented, double-stranded RNA virus that infects nearly all children worldwide by 3–5 years of age; adults can become infected if exposed.
• Reinfections are progressively less severe.
• Large quantities of virus are shed in the stool during the first week of infection, and transmission takes place both via the fecal-oral route and from person to person.
• Disease incidence peaks in the cooler fall and winter months.
After an incubation period of 1–3 days, disease onset is abrupt. Vomiting often precedes diarrhea (loose, watery stools without blood or fecal leukocytes), and about one-third of pts have temperatures >39°C. Symptoms resolve within 3–7 days.
EIAs or viral RNA detection techniques, such as PCR, can identify rotavirus in stool samples.
TREATMENT Rotavirus Infections
Only supportive treatment is needed. Dehydration can be severe, and IV hydration may be needed in pts with frequent vomiting. Avoid antibiotics and antimotility agents.
Rotavirus vaccines, two of which are available, are included in the routine vaccination schedule for U.S. infants. Although these vaccines have a lower efficacy (50–70%) in low-resource settings, the WHO recommends their use in all countries worldwide.
Giardia lamblia (also known as G. intestinalis or G. duodenalis) is a protozoal parasite that inhabits the small intestines of humans and other mammals.
• Cysts are ingested from the environment, excyst in the small intestine, and release flagellated trophozoites that remain in the proximal small intestine. Some trophozoites encyst, with the resulting cysts excreted in feces.
• Transmission occurs via the fecal-oral route, by ingestion of contaminated food and water, or from person to person in settings with poor fecal hygiene (e.g., day-care centers, institutional settings). Infection results from as few as 10 cysts.
• Viable cysts can be eradicated from water by either boiling or filtration. Standard chlorination techniques used to control bacteria do not destroy cysts.
• Young pts, newly exposed pts, and pts with hypogammaglobulinemia are at increased risk—a pattern suggesting a role for humoral immunity in resistance.
After an incubation period of 5 days to 3 weeks, disease ranges from asymptomatic carriage (most common) to fulminant diarrhea and malabsorption.
• Prominent early symptoms include diarrhea, abdominal pain, bloating, belching, flatus, nausea, and vomiting and usually last >1 week. Fever is rare, as is blood or mucus in stool.
• Chronic giardiasis can be continual or episodic; diarrhea may not be prominent, but increased flatulence, sulfurous belching, and weight loss can occur.
• In some cases, disease can be severe, with malabsorption, growth retardation, dehydration, and/or extraintestinal manifestations (e.g., anterior uveitis, arthritis).
Giardiasis can be diagnosed by parasite antigen detection in feces and/or identification of cysts (oval, with four nuclei) or trophozoites (pear-shaped, flattened parasites with two nuclei and four pairs of flagella) in stool specimens. Given variability in cyst excretion, multiple samples may need to be examined.
TREATMENT Giardiasis
• Cure rates with metronidazole (250 mg tid for 5 days) are >90%; tinidazole (2 g PO once) may be more effective. Nitazoxanide (500 mg bid for 3 days) is an alternative agent.
• If symptoms persist, continued infection should be documented before re-treatment and possible sources of reinfection sought. Prolonged therapy with metronidazole (750 mg tid for 21 days) has been successful.
Cryptosporidial infections are caused by Cryptosporidium hominis and C. parvum.
• Oocysts are ingested and subsequently excyst, enter intestinal cells, and generate oocysts that are excreted in feces. The 50% infectious dose in immunocompetent individuals is ~132 oocysts.
• Person-to-person transmission of infectious oocysts can occur among close contacts and in day-care settings. Waterborne transmission is common. Oocysts are not killed by routine chlorination.
After an incubation period of ~1 week, pts may remain asymptomatic or develop watery, nonbloody diarrhea, occasionally with abdominal pain, nausea, anorexia, fever, and/or weight loss lasting 1–2 weeks. In immuno-compromised hosts (particularly those with CD4+ T cell counts <100/μL), diarrhea can be profuse and chronic, resulting in severe dehydration, weight loss, and wasting; the biliary tract can be involved.
On multiple days, fecal samples should be examined for oocysts (4–5 μm in diameter, smaller than most parasites). Modified acid-fast staining, direct immunofluorescent techniques, and EIAs can facilitate diagnosis.
TREATMENT Cryptosporidiosis
• Nitazoxanide (500 mg bid for 3 days) is effective for immunocompetent pts but not for HIV-infected pts; improved immune status due to antiretroviral therapy can alleviate symptoms in the latter pts.
• In addition to antiprotozoal agents, supportive measures include replacement of fluid and electrolytes and use of antidiarrheal agents.
Cystoisospora belli (formerly Isospora belli) infection is acquired by oocyst ingestion and is most common in tropical and subtropical countries. Acute infection can begin suddenly with fever, abdominal pain, and watery, nonbloody diarrhea and can last for weeks or months. Eosinophilia may occur. Compromised (e.g., HIV-infected) pts may have chronic disease that resembles cryptosporidiosis. Detection of large oocysts (~25 μm) in stool by modified acid-fast staining confirms the diagnosis.
TREATMENT Cystoisosporiasis
• Trimethoprim-sulfamethoxazole (TMP-SMX; 160/800 mg bid for 10 days) is effective therapy for immunocompetent pts.
– HIV-infected pts should receive prolonged therapy with TMP-SMX (160/800 mg qid for 10 days, followed by 160/800 mg bid, tid, or qid for 3–4 weeks, depending on the clinical response).
– Pyrimethamine (50–75 mg/d) can be given to pts intolerant of TMP-SMX.
– Pts with AIDS may need suppressive maintenance therapy (TMP-SMX, 160/800 mg 3 times per week) to prevent relapses.
Cyclospora cayetanensis can be transmitted through water or food (e.g., basil, raspberries). Clinical symptoms include diarrhea, flulike symptoms, flatulence, and burping. Disease can be self-limited or can persist for >1 month. Diagnosis is made by detection of oocysts (spherical, 8–10 μm) in stool; targeted diagnostic studies must be specifically requested.
TREATMENT Cyclosporiasis
TMP-SMX (160/800 mg bid for 1 week) is effective. Pts with AIDS may need suppressive maintenance therapy to prevent relapses.
Salmonellae are facultatively anaerobic gram-negative bacilli that cause infection when 103 – 106 organisms are ingested.
• Conditions that reduce gastric acidity or intestinal integrity increase susceptibility to infection.
• Organisms penetrate the small-intestinal mucus layer and traverse the intestinal epithelium through M cells overlying Peyer’s patches.
– S. typhi and S. paratyphi survive within macrophages, then disseminate throughout the body via lymphatics, and ultimately colonize reticuloendothelial tissues.
– Nontyphoidal salmonellae most commonly cause gastroenteritis, invading the large- and small-intestinal mucosa and resulting in massive PMN infiltration (as opposed to the mononuclear-cell infiltration seen with typhoid fever).
Depending on the specific species, salmonellosis results in typhoid fever or gastroenteritis.
• Typhoid (enteric) fever: Typhoid fever is a systemic disease characterized by fever and abdominal pain and caused by dissemination of S. typhi or S. paratyphi, for which humans are the only hosts.
– Disease results from ingestion of food or water contaminated by chronic carriers and is rare in developed nations. Worldwide, there are ~22 million cases, with 200,000 deaths annually.
– After an incubation period of 3–21 days, prolonged fever (>75% of cases), headache (80%), chills (35–45%), anorexia (55%), and abdominal pain (30–40%) are common. Other symptoms may include sweating, cough, malaise, arthralgias, nausea, vomiting, and diarrhea—or, less often, constipation.
– Physical findings include rose spots (faint, salmon-colored, blanching, maculopapular rash), hepatosplenomegaly, epistaxis, and relative bradycardia.
– Intestinal perforation and/or GI hemorrhage can occur in the third and fourth weeks of illness; neurologic manifestations (e.g., meningitis, Guillain-Barré syndrome) occur in 2–40% of pts.
– Long-term Salmonella carriage (i.e., for >1 year) in urine or stool develops in 1–4% of pts.
• Nontyphoidal salmonellosis (NTS): Most commonly caused by S. typhimurium or S. enteritidis, NTS typically presents within 6–48 h of exposure as gastroenteritis (nausea, vomiting, nonbloody diarrhea, abdominal cramping, and fever) that lasts 3–7 days.
– In 2009, there were ~14 million cases of NTS in the United States.
– Disease is acquired from multiple animal reservoirs. The main mode of transmission is via contaminated food products, such as eggs (S. enteritidis), poultry, undercooked meat, dairy products, manufactured or processed foods, and fresh produce. Infection is also acquired during exposure to pets, especially reptiles.
– Stool cultures remain positive for 4–5 weeks and—in rare cases of chronic carriage—for >1 year.
– 8% of pts develop bacteremia, usually due to S. choleraesuis and S. dublin; of these pts, 5–10% develop localized infections (e.g., hepatosplenic abscesses, meningitis, pneumonia, osteomyelitis).
– Reactive arthritis can follow Salmonella gastroenteritis, particularly in persons with the HLA-B27 histocompatibility antigen.
Positive cultures of blood, stool, or other specimens are required for diagnosis.
TREATMENT Salmonellosis
• Typhoid fever: A fluoroquinolone (e.g., ciprofloxacin, 500 mg PO bid) is most effective for susceptible organisms.
– Pts infected with nalidixic acid–resistant strains (whose susceptibility to ciprofloxacin is reduced) should be treated with ceftriaxone (2–3 g/d IV for 7–14 days), azithromycin (1 g/d PO for 5 days), or high-dose ciprofloxacin (750 mg PO bid or 400 mg IV q8h for 10–14 days).
– Dexamethasone may be of benefit in severe cases.
• NTS: Antibiotic treatment is not recommended in most cases as it does not shorten the duration of symptoms and is associated with increased rates of relapse, a prolonged carrier state, and adverse drug reactions.
– Antibiotic treatment may be required for infants ≤3 months of age; pts >50 years of age with suspected atherosclerosis; pts with immunosuppression; pts with cardiac, valvular, or endovascular abnormalities; and pts with significant joint disease.
– Fluoroquinolones or third-generation cephalosporins are given for 2–3 days or until defervescence (if the pt is immunocompe-tent) or for 1–2 weeks (if the pt is immunocompromised).
– HIV-infected pts are at high risk for Salmonella bacteremia and should receive 4 weeks of oral fluoroquinolone therapy after 1–2 weeks of IV treatment. In cases of relapse, long-term suppression with a fluoroquinolone or TMP-SMX should be considered.
– Pts with endovascular infections or endocarditis should receive 6 weeks of treatment with a third-generation cephalosporin.
Campylobacters are motile, curved gram-negative rods that are a common bacterial cause of gastroenteritis in the United States. Most cases are caused by C. jejuni.
Campylobacters are common commensals in the GI tract of many food animals and household pets. In the United States, ingestion of contaminated poultry accounts for 30–70% of cases. Transmission to humans occurs via contact with or ingestion of raw or undercooked food products or direct contact with infected animals.
An incubation period of 2–4 days (range, 1–7 days) is followed by a prodrome of fever, headache, myalgia, and/or malaise. Within the next 12–48 h, diarrhea (with stools containing blood, mucus, and leukocytes), cramping abdominal pain, and fever develop.
• Most cases are self-limited, but illness persists for >1 week in 10–20% of pts and may be confused with inflammatory bowel disease.
• Species other than C. jejuni (e.g., C. fetus) can cause a similar illness or prolonged relapsing systemic disease without a primary focus in immunocompromised pts.
– The course may be fulminant, with bacterial seeding of many organs, particularly vascular sites.
– Fetal death can result from infection in a pregnant pt.
• Three patterns of extraintestinal infection have been noted: (1) transient bacteremia in a normal host with enteritis (benign course, no specific treatment needed); (2) sustained bacteremia or focal infection in a normal host; and (3) sustained bacteremia or focal infection in a compromised host.
• Complications include reactive arthritis (particularly in persons with the HLA-B27 phenotype) and Guillain-Barré syndrome (in which campylobacters are associated with 20–40% of cases).
The diagnosis is confirmed by cultures of stool, blood, or other specimens on special media and/or with selective techniques.
TREATMENT Campylobacteriosis
• Fluid and electrolyte replacement is the mainstay of therapy.
• Use of antimotility agents is not recommended, as they are associated with toxic megacolon.
• Antibiotic treatment (erythromycin, 250 mg PO qid for 5–7 days) should be reserved for pts with high fever, bloody or severe diarrhea, persistence for >1 week, and worsening of symptoms. Azithromycin and fluoroquinolones are alternative regimens, although resistance to these drugs is increasing.
Shigellae are small, gram-negative, nonmotile bacilli that are very closely related to E. coli. The four most common Shigella serotypes are S. dysenteriae type 1, S. flexneri, S. boydii, and S. sonnei (which is more prevalent in the industrialized world). There are no animal reservoirs other than higher primates.
• These bacteria are transmitted from person to person via the fecal-oral route and occasionally via intermediate vectors such as food and water.
• The ability of as few as 100 organisms to cause infection helps explain the high rate of secondary household transmission.
• Shiga toxin and Shiga-like toxins produced by some strains of E. coli (including O157:H7) are important factors in disease severity. The toxins target endothelial cells and play a significant role in the microangiopathic complications of Shigella and E. coli infections, such as hemolyticuremic syndrome (HUS) and thrombotic thrombocytopenic purpura.
• An analysis of cases occurring in 1966–1997 revealed an incidence of 165 million cases (of which 69% affected children <5 years of age) with 0.5–1.1 million deaths annually; these numbers have likely decreased since then, but multidrug-resistant strains have emerged.
After an incubation period of 1–4 days, shigellosis evolves through three phases: watery diarrhea, dysentery (bloody mucopurulent stools), and the postinfectious phase.
• Most episodes resolve in 1 week without treatment; with appropriate treatment, recovery takes place within a few days, with no sequelae.
• Complications are largely intestinal (e.g., toxic megacolon, intestinal perforation, rectal prolapse) or metabolic (e.g., hypoglycemia, hyponatremia). Shiga toxin produced by S. dysenteriae type 1 is linked to HUS (Coombs-negative hemolytic anemia; thrombocytopenia; and acute renal failure) in developing countries but is rare in industrialized countries, where E. coli O157:H7 is a more common cause.
Shigellosis is diagnosed directly by stool culture. STEC/EHEC infection is diagnosed by screening of stool cultures for E. coli strains that do not ferment sorbitol, with subsequent serotyping for O157. Tests to detect Shiga toxins or toxin genes are sensitive, specific, and rapid; this approach detects non-O157 STEC/EHEC and sorbitol-fermenting strains of O157:H7.
TREATMENT Shigellosis and Infection with STEC/EHEC
• In the United States, because of the ready transmissibility of Shigella, antibiotics are recommended. Fluoroquinolones (e.g., ciprofloxacin, 500 mg bid) are effective, as are ceftriaxone, azithromycin, and pivmecillinam.
– S. dysenteriae infection should be treated for 5 days and non- dysenteriae Shigella infection for 3 days.
– Immunocompromised pts should receive 7–10 days of treatment.
• Antibiotic treatment for STEC/EHEC infections should be avoided, since antibiotics may increase the incidence of HUS.
• Rehydration usually is not needed; Shigella infection rarely causes significant dehydration. If required, rehydration should be oral, and nutrition should be started as soon as possible. Use of antimotility agents may prolong fever and increase the risk of HUS and toxic megacolon.
Y. enterocolitica and Y. pseudotuberculosis are nonmotile gram-negative rods that cause enteritis or enterocolitis with self-limited diarrhea that lasts an average of 2 weeks as well as mesenteric adenitis (especially common with Y. pseudotuberculosis) and terminal ileitis (especially common with Y. enterocolitica) that can resemble acute appendicitis. Septicemia can occur in pts with chronic liver disease, malignancy, diabetes mellitus, and other underlying illnesses. Infection has been linked to reactive arthritis in HLA-B27-positive pts.
Stool culture studies for Yersinia must be specifically requested and require the use of special media.
TREATMENT Yersiniosis
Antibiotics are not indicated for diarrhea caused by yersiniae; supportive measures suffice.
Caused by Entamoeba histolytica, amebiasis has a high incidence in developing countries and among travelers, recent immigrants, men who have sex with men, and inmates of institutions in developed nations. Infection follows ingestion of cysts from fecally contaminated water, food, or hands. Motile trophozoites are released from cysts in the small intestine and then cause infection in the large bowel. Trophozoites may be shed in stool (in active dysentery) or encyst. Excreted cysts survive for weeks in a moist environment.
Most pts harboring Entamoeba species are asymptomatic, but some pts develop inflammatory colitis 2–6 weeks after ingestion of amebic cysts.
• Dysentery may develop, with daily passage of 10–12 small stools consisting mostly of blood and mucus. Fewer than 40% of pts have fever.
• Fulminant amebic colitis—characterized by more profuse diarrhea, severe abdominal pain with peritoneal signs, and fever—is more common among young children, pregnant women, and pts taking glucocorticoids.
• Liver abscess is the most common type of extraintestinal infection and can arise months or years after exposure to E. histolytica. Pts present with right-upper-quadrant pain, fever, right-sided pleural effusion, and hepatic tenderness and typically do not have active colitis. The abscess can rupture through the diaphragm and metastasize elsewhere (e.g., lung, brain).
Microscopic examination of three stool samples, often combined with serologic testing, remains the standard diagnostic approach.
• Radiographic demonstration of at least one space-occupying lesion in the liver combined with positive serology confirms the diagnosis of amebic liver abscess. In this setting, serology has a sensitivity of >94% and a specificity of >95%.
TREATMENT Amebiasis
• Tinidazole (2 g/d PO for 3 days) or metronidazole (750 mg PO or IV tid for 5–10 days) is recommended for amebic colitis and amebic liver abscess.
– >90% of pts respond clinically within 3 days of treatment initiation.
– Drainage of liver abscesses is rarely needed. Indications for aspiration include the need to rule out pyogenic abscess, a lack of response to treatment after 4 days, an imminent threat of liver-abscess rupture, or the need to prevent left-lobe abscess rupture into the pericardium.
• Pts with either colitis or liver abscesses should also receive a luminal agent to ensure eradication of the infection. Paromomycin (10 mg/kg PO tid for 5–10 days) is the preferred agent; iodoquinol (650 mg PO tid for 20 days) is an alternative.
C. difficile is an obligately anaerobic, gram-positive, spore-forming bacillus and causes diarrheal illness that is most commonly acquired in the hospital. The disease is acquired almost exclusively in association with antimicrobial treatment; virtually all antibiotics carry a risk of CDI.
• After C. difficile colonizes the gut, its spores vegetate, multiply, and secrete toxin A (an enterotoxin) and toxin B (a cytotoxin), causing diarrhea and pseudomembranous colitis. The rate of fecal colonization is often ≥20% among adult pts hospitalized for >1 week; in contrast, the rate is 1–3% among community residents.
• Spores can persist on environmental surfaces in the hospital for months and on the hands of hospital personnel who do not practice adequate hand hygiene.
• Rates and severity of CDI in the United States, Canada, and Europe have increased markedly in the past decade. An epidemic strain accounts for much of the increase and is characterized by production of 16–23 times as much toxin A and toxin B as is documented for control strains, by the presence of a third toxin (binary toxin), and by high-level resistance to fluoroquinolones.
Most commonly, pts develop diarrhea, with stools that are not grossly bloody and are soft to watery, with a characteristic odor. Pts may have up to 20 bowel movements per day. Fever, abdominal pain, and leukocytosis are common.
• Constipation due to an adynamic ileus can occur. Unexplained leukocytosis (≥15,000 WBCs/μL) in this setting should prompt evaluation for CDI. These pts are at high risk for complications such as toxic megacolon and sepsis.
• C. difficile diarrhea recurs after treatment in ~15–30% of cases.
The diagnosis of CDI is made in a pt with diarrhea (≥3 unformed stools per 24 h for ≥2 days) by detection of the organism, toxin A, or toxin B in stool or identification of pseudomembranes in the colon.
• Most laboratory tests for toxin lack sensitivity, but repeat testing is not recommended.
• Testing of asymptomatic pts (including a test of cure for those who have completed therapy) is not recommended.
TREATMENT C. difficile Infection
• Primary CDI: When feasible, discontinuation of ongoing antimicrobial treatment is an effective cure in 15–23% of cases. Prompt initiation of specific therapy is recommended.
– For mild to moderate disease, metronidazole (500 mg tid for 10 days) is recommended, with extension of therapy if the clinical response is slow.
– For severe disease (e.g., >15,000 WBCs/μL, serum creatinine levels ≥1.5 times baseline), vancomycin (125 mg qid PO for 10–14 d) is the agent of choice.
• Recurrent CDI: The first recurrence should be treated the same as the initial episode.
– For the second recurrence, an extended, tapered vancomycin regimen (125 mg qid for 10–14 d, then bid for 1 week, then daily for 1 week, then q2–3d for 2–8 weeks) should be used.
– For multiple recurrences, there is no standard treatment course. Consider repetition of the tapered vancomycin regimen, administration of vancomycin (500 mg qid for 10 days) with Saccharomyces boulardii (500 mg bid for 28 d), administration of sequential therapy with vancomycin (125 mg qid for 10–14 d) followed by rifaximin (400 mg bid for 2 weeks), treatment with nitazoxanide (500 mg bid for 10 d), fecal transplantation, or treatment with IV immunoglobulin (400 mg/kg).
• Fulminant CDI: Medical management is complicated by ineffective delivery of oral antibiotics to the intestinal lumen in the setting of ileus. Vancomycin (given via nasogastric tube and by retention enema) combined with IV metronidazole has been used with some success, as has IV tigecycline. Surgical colectomy can be life-saving.

For a more detailed discussion, see LaRocque RC, Ryan ET, Calderwood SB: Acute Infectious Diarrheal Diseases and Bacterial Food Poisoning, Chap. 128, p. 1084; Gerding DN, Johnson S: Clostridium difficile Infection, Including Pseudomembranous Colitis, Chap. 129, p. 1091; Russo TA, Johnson JR: Diseases Caused by Gram-Negative Enteric Bacilli, Chap. 149, p. 1246; Pegues DA, Miller SI: Salmonellosis, Chap. 153, p. 1274; Sansonetti P, Bergounioux J: Shigellosis, Chap. 154, p. 1281; Blaser MJ: Infections Due to Campylobacter and Related Organisms, Chap. 155, p. 1286; Waldor MK, Ryan ET: Cholera and Other Vibrioses, Chap. 156, p. 1289; Prentice MB: Plague and Other Yersinia Infections, Chap. 159, p. 1305; Parashar UD, Glass RI: Viral Gastroenteritis, Chap. 190, p. 1588; Stanley SL Jr: Amebiasis and Infection With Free-Living Amebas, Chap. 209, p. 1683; and Weller PF: Protozoal Intestinal Infections and Trichomoniasis, Chap. 215, p. 1729, in HPIM-18.