Chapter 2
What Your Body Does All Day
IN THIS CHAPTER
 Seeing what your body does automatically every day
Seeing what your body does automatically every day
 Finding out what goes on inside of every cell
Finding out what goes on inside of every cell
 Discovering the importance of homeostasis
Discovering the importance of homeostasis
 Building and maintaining your parts
Building and maintaining your parts
This chapter is about your life as an organism. As Chapter 1 explains, organism is the fifth of five levels of organization in living things. Although the word organism has many possible definitions, for the purposes of this chapter, an organism is a living unit that metabolizes and maintains its own existence.
In this chapter, you see why your to-do list, crowded as it is, doesn’t include items such as Take ten breaths every minute or At 11:30 a.m., open sweat glands. The processes that your body must carry out minute by minute to sustain life, not to mention the biochemical reactions that happen millions of times a second, can’t be left to the distractible frontal lobes (the conscious, planning part of your brain). Instead, your organs and organ systems function together smoothly to carry out these processes and reactions automatically, without the activity ever coming to your conscious attention. All day and all night, year in and year out, your body builds, maintains, and sustains every part of you; keeps your temperature and your fluid content within some fairly precisely defined ranges; and transfers substances from outside itself to inside, and then back out again. These are the processes of metabolism and homeostasis.
Transferring Energy: A Body’s Place in the World
The laws of thermodynamics are the foundation of how the physics and chemistry of the universe are understood. They’re at the “we hold these truths to be self-evident” level for chemists and physicists of all specialties, including all biologists. The first law of thermodynamics states that energy can be neither created nor destroyed — it can only change form. (Turn to Chapter 16 for a brief look at the first law and other basic laws of chemistry and physics.) Energy changes form continuously — within stars, within engines of all kinds, and, in some very special ways, within organisms.
The most basic function of the organism that is you on this planet is to take part in this continuous flow of energy. As a heterotroph (an organism that doesn’t photosynthesize), you ingest (take in) energy in the form of matter — that is, you eat the bodies of other organisms. You use the energy stored in the chemical bonds of that matter to fuel the processes of your metabolism and homeostasis. That energy is thereby transformed into matter called “you” (the material in your cells), matter that’s “not you” (the material in your exhaled breath and in your urine), and some heat radiated from your body to the environment.
 Hetero means “other,” and tropho means “nourishment.” A heterotroph gets its nourishment from others, as opposed to an autotroph, which makes its own nourishment, as a plant does.
Hetero means “other,” and tropho means “nourishment.” A heterotroph gets its nourishment from others, as opposed to an autotroph, which makes its own nourishment, as a plant does.
Plants convert light energy from the sun into the chemical energy in carbohydrates, which comprise most of the matter of the plant bodies, recycling the waste matter (carbon dioxide) of your metabolic processes. Energy goes around and around, and some of it is always flowing through your body, being transformed constantly as it does so. You, my friend, are part of a cycle of cosmic dimensions!
Building Up and Breaking Down: Metabolism
The word metabolism describes all the chemical reactions that happen in the body. These reactions are of two kinds — anabolic reactions make things (molecules), and catabolic reactions break things down.
 To keep the meanings of anabolic and catabolic clear in your mind, associate the word catabolic with the word catastrophic to remember that catabolic reactions break down products. Then you’ll know that anabolic reactions create products.
To keep the meanings of anabolic and catabolic clear in your mind, associate the word catabolic with the word catastrophic to remember that catabolic reactions break down products. Then you’ll know that anabolic reactions create products.
Your body performs both anabolic and catabolic reactions at the same time, around the clock, to keep you alive and functioning. Even when you’re sleeping, your cells are busy. You just never get to rest (until you’re dead).
Chapter 11 gives you the details on how the digestive system breaks down food into nutrients and gets them into your bloodstream. Chapter 9 explains how the bloodstream carries nutrients around the body to every cell and carries waste products to the urinary system. Chapter 12 shows you how the urinary system filters the blood and removes waste from the body. This chapter describes the reactions that your cells undergo to convert fuel to usable energy. Ready?
Why your cells metabolize
Even when your outside is staying still, your insides are moving. Day and night, your muscles twitch and contract and maintain “tone.” Your heart beats. Your blood circulates. Your diaphragm moves up and down with every breath. Nervous impulses travel. Your brain keeps tabs on everything. You think. Even when you’re asleep, you dream (a form of thinking). Your intestines push the food you ate hours ago along your alimentary canal. Your kidneys filter your blood and make urine. Your sweat glands open and close. Your eyes blink, and even during sleep, they move. Men produce sperm. Women move through the menstrual cycle. The processes that keep you alive are always active.
Every cell in your body is like a tiny factory, converting raw materials to useful molecules such as proteins and thousands of other products, many of which we discuss throughout this book. The raw materials (nutrients) come from the food you eat, and the cells use the nutrients in metabolic reactions. During these reactions, some of the energy from catabolized nutrients is used to generate a compound called adenosine triphosphate (ATP). This molecule is the one your cells can actually use to power all those chemical reactions.
 So, nutrients are catabolized (broken down), ATP is formed (anabolized), and when needed, ATP is catabolized (for energy). This principle of linked anabolic and catabolic reactions is one of the cornerstones of human physiology and is required to maintain life. Cellular metabolism also makes waste products that must be removed (exported) from the cell and ultimately from the body.
So, nutrients are catabolized (broken down), ATP is formed (anabolized), and when needed, ATP is catabolized (for energy). This principle of linked anabolic and catabolic reactions is one of the cornerstones of human physiology and is required to maintain life. Cellular metabolism also makes waste products that must be removed (exported) from the cell and ultimately from the body.
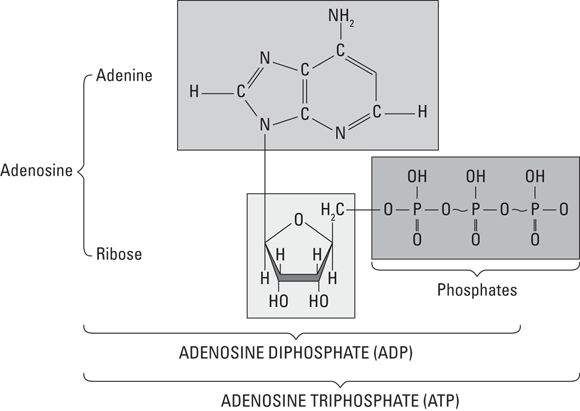
ATP works like a rechargeable battery. It contains three phosphates aligned in a row (see Figure 2-1). Breaking one of them off accesses the energy, leaving behind adenosine diphosphate (ADP) and a phosphate (P) by itself. However, just like you can plug in your phone to recharge its battery, the energy in the bonds of glucose is used to reattach the P — re-creating ATP (albeit in an incredibly complicated way).
How your cells metabolize
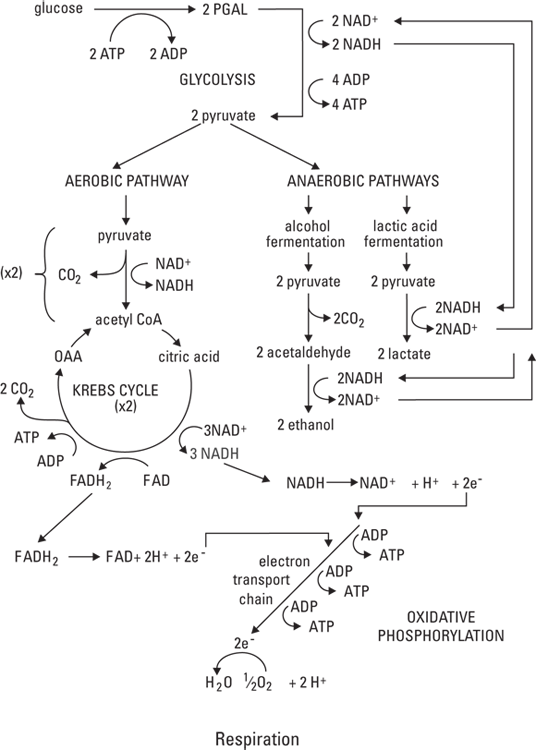
The reactions that convert fuel (specifically glucose) to usable energy (ATP molecules) include glycolysis, the Krebs cycle (aerobic respiration) and anaerobic respiration, and oxidative phosphorylation. Together, these reactions are referred to as cellular respiration. These are complex pathways, so expect to take some time to understand them. See Figure 2-2 and refer to it as many times as necessary to understand what happens in cellular respiration. (Note: Alcohol fermentation is included for reference but does not occur in the human body.)
Here, we focus on the ins and outs of the three main components of cellular respiration.
Glycolytic pathway (glycolysis)
Starting at the top of Figure 2-2, you can see that glucose — the smallest molecule that a carbohydrate can be broken into during digestion — goes through the process of glycolysis, which starts cellular respiration and uses some energy (ATP) itself. Glycolysis occurs in the cytoplasm and doesn’t require oxygen. Two molecules of ATP are required to start each molecule of glucose rolling down the glycolytic pathway; although four molecules of ATP are generated during glycolysis, the net production of ATP is two molecules. In addition to the two ATPs, two molecules of pyruvic acid (also called pyruvate) are generated. They move into a mitochondrion and enter the Krebs cycle.
Krebs cycle
The Krebs cycle is a major biological pathway in the metabolism of every multicellular organism. It’s an aerobic pathway, requiring oxygen.
As the pyruvate enters the mitochondrion, a molecule called nicotinamide adenine dinucleotide (NAD+) joins it. NAD+ is an electron carrier (that is, it carries energy), and it gets the process moving by bringing some energy into the pathway. The NAD+ provides enough energy that when it joins with pyruvate, carbon dioxide is released, and the high-energy molecule NADH is formed. Flavin adenine dinucleotide (FAD) works in much the same way, becoming FADH2. The product of the overall reaction is acetyl coenzyme A (acetyl CoA), which is a carbohydrate molecule that puts the Krebs cycle in motion.
 Cycles are endless. Products of some reactions in the cycle are used to keep the cycle going. An example is acetyl CoA: It’s a product of the Krebs cycle, yet it also helps initiate the cycle. With the addition of water and acetyl CoA, oxaloacetic acid (OAA) is converted to citric acid. Then, a series of reactions proceeds throughout the cycle.
Cycles are endless. Products of some reactions in the cycle are used to keep the cycle going. An example is acetyl CoA: It’s a product of the Krebs cycle, yet it also helps initiate the cycle. With the addition of water and acetyl CoA, oxaloacetic acid (OAA) is converted to citric acid. Then, a series of reactions proceeds throughout the cycle.
Oxidative phosphorylation
Oxidative phosphorylation, also called the electron transport chain (ETC), takes place in the inner membrane of the mitochondria. The electron carriers produced during the Krebs cycle — NADH and FADH2 — are created when NAD+ and FAD, respectively, are “reduced.” When a substance is reduced, it gains electrons; when it’s oxidized, it loses electrons. (Turn to Chapter 16 for more information about such “redox reactions.”) So NADH and FADH2 are compounds that have gained electrons, and therefore, energy. In the ETC, oxidation and reduction reactions occur repeatedly as a way of transporting energy. At the end of the chain, oxygen atoms accept the electrons, producing water. (Water from metabolic reactions isn’t a significant contributor to the water needs of the body.)
As NADH and FADH2 pass down the respiratory (or electron transport) chain, they lose energy as they become oxidized and reduced, oxidized and reduced, oxidized and … . It sounds exhausting, doesn’t it? Well, their energy supplies become exhausted for a good cause. The energy that these electron carriers lose is used to add a molecule of phosphate to adenosine diphosphate (ADP) to make it adenosine triphosphate — the coveted ATP. For each NADH molecule that’s produced in the Krebs cycle, three molecules of ATP can be generated. For each molecule of FADH2 that’s produced in the Krebs cycle, two molecules of ATP are made.
 Theoretically, the entire process of aerobic cellular respiration — glycolysis, Krebs cycle, and oxidative phosphorylation — generates a total of 38 ATP molecules from the energy in one molecule of glucose: 2 from glycolysis, 2 from the Krebs cycle, and 34 from oxidative phosphorylation. However, this theoretical yield is never quite reached because processes, especially biological processes, are never 100 percent efficient. In the real world, usually around 29 to 30 ATP molecules per glucose molecule are expected.
Theoretically, the entire process of aerobic cellular respiration — glycolysis, Krebs cycle, and oxidative phosphorylation — generates a total of 38 ATP molecules from the energy in one molecule of glucose: 2 from glycolysis, 2 from the Krebs cycle, and 34 from oxidative phosphorylation. However, this theoretical yield is never quite reached because processes, especially biological processes, are never 100 percent efficient. In the real world, usually around 29 to 30 ATP molecules per glucose molecule are expected.
Anaerobic respiration
Sometimes oxygen isn’t present, but your body still needs energy. During these times, a backup system, an anaerobic pathway (called anaerobic because it proceeds in the absence of oxygen) exists. Lactic acid fermentation generates NAD+ so that glycolysis, which results in the net production of two molecules of ATP, can continue. However, if the supply of NAD+ runs out, glycolysis can’t occur, and ATP can’t be generated.
This occurs most often in muscle cells during periods of intense exercise. The byproduct of this reaction, lactic acid, builds up in the muscle, contributing to muscle fatigue (the inability of a muscle cell to contract). Thus, this process cannot be maintained for extended periods of time.
Staying in Range: Homeostasis
Chemical reactions are not random events. Any reaction takes place only when all the conditions are right for it: All the required reagents and catalysts are close together in the right quantities; the fuel for the reaction is present, in sufficient amount and in the right form; and the environmental variables are all within the right range, including the temperature, salinity, and pH. The complicated chemistry of life is extremely sensitive to the environmental conditions; the environment being the body itself. Homeostasis is the term physiologists use to reference the balance of all the variables. Numerous homeostatic mechanisms are employed by our bodies to keep everything in check; otherwise, all the reactions that comprise our metabolism cannot occur.
The following sections look at a few important physiological variables and how the mechanisms of homeostasis keep them in the optimum range in common, everyday situations.
 As metabolic reactions, homeostatic reactions require energy.
As metabolic reactions, homeostatic reactions require energy.
Maintaining a constant temperature: Thermoregulation
All metabolic reactions in all organisms require that the temperature of the body be within a certain range. Because we humans are homeotherms or “warm-blooded,” we maintain a relatively constant body temperature regardless of the ambient temperature. We do this by regulating our metabolic rate. The large number of mitochondria per cell enables a high rate of metabolism, which generates a lot of heat.
 Regulating body temperature requires a steady supply of fuel (glucose) to the mitochondrial furnaces.
Regulating body temperature requires a steady supply of fuel (glucose) to the mitochondrial furnaces.
Another way we control our body temperature is by employing adaptations that conserve the heat generated by metabolism within the body in cold conditions or dissipate that heat out of the body in overly warm conditions. A few of the specific adaptations include the following:
- Sweating: Sweat glands in the skin open their pores to dissipate heat by evaporative cooling of water from the skin. They close to conserve heat. Sweat glands are opened and closed by the action of muscles at the base of the gland, deep under the skin. Refer to Chapter 4.
- Blood circulation: Blood vessels close to the skin dilate (enlarge) to dissipate heat in the blood through the skin. They constrict (narrow) to conserve heat. That’s why your skin flushes (reddens) when you’re hot: That’s the color of your blood visible at the surface of your skin. Refer to Chapter 9.
- Muscle contraction: When closing sweat pores and blood vessel constriction are not enough to conserve heat in cold conditions, your muscles will begin to contract automatically to generate more heat. This reaction is familiar as “shivering.”
- Insulation: Regions of fatty tissue under the skin provide insulation, holding the warmth of the body in. Body hair aids in this, too (though not enough to keep us from needing a nice, warm winter coat).
Swimming in H2O: Fluid balance
A watery environment is a requirement for a great proportion of metabolic reactions (the rest need a lipid, or fatty, environment). The body contains a lot of water: in your blood, in your cells, in the spaces between your cells, in your digestive organs, here, there, and everywhere. Not pure water, though. The water in your body is a solvent for thousands of different ions and molecules (solutes). The quantity and quality of the solutes change the character of the solution. Because solutes are constantly entering and leaving the solution as they participate in or are generated by metabolic reactions, the characteristics of the watery solution must remain within certain bounds for the reactions to continue happening.
- Changes in the composition of urine: The kidney is a complex organ that has the ability to measure the concentration of many solutes in the blood, including sodium, potassium, and calcium. Very importantly, the kidney can measure the volume of water in the body by sensing the pressure of the blood as it flows through (the greater the volume of water, the higher the blood pressure). If changes must be made to bring the volume and composition of the blood back into the ideal range, the various structures of the kidney incorporate more or less water, sodium, potassium, and so on into the urine. That’s why your urine is paler or darker at different times. This and other functions of the urinary system are discussed in Chapter 12.
- The thirst reflex: Water passes through your body constantly: mainly, in through your mouth and out through various organ systems, including the skin, the digestive system, and the urinary system. If the volume of water falls below the optimum level (dehydration), and the kidneys alone can’t regain the balance, the mechanisms of homeostasis intrude on your conscious brain to make you uncomfortable. You feel thirsty. You ingest something watery. Your fluid balance is restored and your thirst reflex leaves you alone.
Adjusting the fuel supply: Blood glucose concentration
Glucose, the fuel of all cellular processes, is distributed to all cells dissolved in the blood. The concentration of glucose in the blood must be high enough to ensure that the cells have enough fuel. However, extra glucose beyond the immediate needs of the cells can harm many important organs and tissues, especially where the vessels are tiny, as in the retina of the eye, the extremities (hands and, especially, feet), and the kidneys. Diabetes is a disease in which there is a chronic overconcentration of glucose in the blood.
The amount of glucose in the blood is controlled mainly by the pancreas. Absorption by the small intestine puts the glucose from ingested food into the blood. Insulin is a hormone released into the blood from the pancreas in response to increased blood glucose levels. Most cells have receptors that bind the insulin, which allows glucose into the cells for cellular respiration. The cells of the liver, muscles, and adipose tissue (fat) take up the glucose and store it as glycogen (see Chapter 3). At times when your intestines aren’t absorbing much glucose, like hours after a meal, the production of insulin is suppressed and the stored glucose is released into the blood again. Refer to Chapter 8 for more information about the pancreatic control of blood glucose levels.
Measuring important variables
How does the pancreas know when to release insulin and how much is enough? How does the kidney know when the salt content of the blood is too high or the volume of the blood is too low? What tells the sweat glands to open and close to cool the body or retain heat? Well, read on!
Every homeostatic mechanism employs three parts: a receptor, an integrator, and an effector. Numerous receptors, or sensors, are strategically placed throughout your body. Some respond to chemical changes (like pH), others to mechanical ones (like blood pressure), and there are many more. These receptors are specialized nervous cells and communicate to the brain — the integrator — any changes from our balance. The brain processes all the incoming information and “decides” if a response is warranted. If it is, a message will be sent out via neurons or hormones to the effectors, which carry out the body’s response (the effect).
Growing, Replacing, and Renewing
My, how you’ve changed, and are still changing! Growing up, growing old, and just living every day, you’re building new parts and replacing old ones. From conception to early adulthood, your body was busy making itself: everything from scratch.
But the job wasn’t finished when you were fully grown. Complex living tissues and organs almost all require replacement parts at some time, and many require them all the time. This necessity is one of the defining characteristics of organisms — the ability to organize matter into the structures that compose themselves and to replace and renew those structures as required, as we describe in the following sections.
 As we discuss in the “Building Up and Breaking Down: Metabolism” section earlier in the chapter, making new cells and tissues is anabolic metabolism, and breaking up and eliminating old cells and tissues is catabolic metabolism.
As we discuss in the “Building Up and Breaking Down: Metabolism” section earlier in the chapter, making new cells and tissues is anabolic metabolism, and breaking up and eliminating old cells and tissues is catabolic metabolism.
Growing
You began life as a single cell and built yourself from there, with some help from your mom to get started. Your body developed along a plan, building a backbone with a head at the top and a tail at the bottom (somehow, you lost the tail). Now look at you: 100 trillion cells, almost every one with its own special structure and job to do. Good work! Find more about the processes of development in Chapter 15.
Replacing
Just like the organism they’re a part of, many kinds of cells have a life cycle: They’re born, they develop, they work, they get worn out, and they die. For an organism to continue its life cycle, these cells must be replaced continuously, either by the division of the same cell type or by the differentiation of stem cells. These relatively undifferentiated cells wait patiently until they’re called upon to divide. Some of the daughter cells differentiate into their specific programmed type while others remain stem cells and wait to be called upon the next time. Stem cells are an active area of research in physiology and in the field of regenerative medicine.
Some of the cell and tissue types that must be continuously replaced are
- Red blood cells: The life cycle of a red blood cell is about 120 days. That means you replace all your red blood cells three times a year. New ones come from the red marrow of the bones, and old ones are scavenged for iron in the spleen and then broken down in the liver.
- Epidermis: The cells of the epidermis, the outer layer of the skin, are constantly shed from the surface and replaced from below. Your body replaces the entire epidermis about every six weeks. This process is discussed in Chapter 4.
- Intestinal lining: The epithelial cells of the intestinal lining are replaced about every week. You realize what a feat this is when you read about the intestine in Chapter 11.
- Respiratory membrane: Your body replaces the epithelial cells that line the alveolar wall and the pulmonary capillary vessels about every week. Refer to Chapter 10 for a description of the respiratory membrane.
- Sperm: The process of spermatogenesis (making sperm) is continuous, beginning in a male’s puberty and ending with his death. The quantity and quality varies with his age and health. Turn to Chapter 14 for more details.
- Bone: As you can read about in Chapter 5, bone is living tissue and very active in a number of ways. The bones bear the body’s weight and the stress of impact. Tiny cracks develop in bone all the time and are repaired quickly and constantly, a process known as remodeling. Bones serve as a storage depot for metal ions, especially calcium, which flows in and out of the bone constantly.
Some other types of tissue replace their cells at a very slow rate, such as the following:
- Brain cells: Scientists thought for many decades that brain cells that died weren’t replaced, and, in general, that no new cells were developed in the brain during adulthood. Brain researchers have now shown that this isn’t true. Still, neurons are designed to not need replacement, they’re meant to last you a lifetime. The processes whereby new cells are born in the adult brain have attracted much research interest. See Chapter 7.
- Cardiac muscle: Until recently, physiologists believed that cardiac muscle cells couldn’t regenerate, but that belief has recently been called into question. In 2009, researchers in Sweden reported evidence that, in healthy hearts, cardiac muscle cells do indeed divide, but slowly. The researchers estimated that a 20-year-old renews about 1 percent of heart muscle cells per year and that about 45 percent of the cardiac muscle cells of a 50-year-old are generated after birth. Research published in the early 2000s showed evidence that cardiac muscle cells regenerate to some extent after a heart attack.
Repairing parts
Your body repairs some tissues as necessary, such as after an injury:
- Skeletal muscle: Mature skeletal muscle cells, called fibers, don’t divide and aren’t replaced unless they’re damaged. After they’re formed, skeletal muscle fibers generally survive for your entire lifetime. But wait, you say that you’ve been working out and your biceps are twice as big as they were last year? Congratulations, but you didn’t add cells. The cells you had just got bigger.
- Smooth muscle: Like skeletal muscle fibers, smooth muscle fibers are replaced when they’re injured.
- Skin fibroblasts: These are different from epidermal cells. These cells proliferate rapidly to repair damage from a cut or wound and are responsible for generating scar tissue, as discussed in the next section.
- Liver cells: Normally, these cells divide only rarely. However, if large numbers of liver cells are removed — by surgical removal of part of the liver, for example — the remaining cells proliferate rapidly to replace the missing tissue. This makes it possible to transplant part of the liver of a living donor to a recipient, or to split a single liver from a nonliving donor to two recipients. In these cases, when all goes well, both parts regenerate into a complete and functioning liver.
Healing wounds
When you have a tiny, superficial surface wound (a little scratch), the epidermis simply replaces the damaged cells. In a few days, the scratch is gone. But when the wound is deep enough that blood vessels are damaged, the healing process is a little bit more involved. Turn to Chapter 9 for information on blood and blood vessels.
The immediate rush of blood washes debris and microbes out of the wound. Then, the vessels around the wound constrict to slow down the blood flow. A type of “formed element” in the blood called platelets sticks to the collagen fibers that make up the vessel wall, forming a natural band-aid called a platelet plug.
After the platelet plug forms, a complex chain of events results in the formation of a clot that stops blood loss altogether. This chain of events is called the clotting cascade or coagulation cascade. Enzymes called clotting factors initiate the cascade. Here’s a rundown of what happens, focusing on the most important steps:
- Prothrombin: This clotting factor converts to thrombin. Calcium is required for this reaction.
- Thrombin: This factor acts as an enzyme and causes the plasma protein fibrinogen to form long threads called fibrin.
- Fibrin threads: Wrapping around the platelet plug, these threads form a meshlike template for a clot.
- Clot: The meshlike structure traps the red blood cells and forms a clot. As the red blood cells that are trapped on the outside of the clot dry out (or the air oxidizes the iron in them, like rust), they turn a brownish-red color, and a scab forms.
Underneath the scab, the blood vessels regenerate and repair themselves, and in the dermis, cells called fibroblasts spur the creation of proteins to fill the space in the damaged layers. Scars are created to provide extra strength to skin areas that are deeply wounded. Scar tissue has many interwoven collagen fibers, but no hair follicles, nails, or glands. Feeling maybe lost in the area covered with scar tissue if the nerves are damaged.
Lasting parts
As we mention earlier in the chapter, almost all tissues and organs require replacement parts at some time. However, here are some exceptions:
- Central nervous system: For the most part, the cells and tissues of the central nervous system are incapable of self-repair and regeneration. Thus the poor prognosis in cases of spinal cord injury.
- Peripheral nerves: These are the nerve cells that transmit sensation or motor messages between the central nervous system and the skin and skeletal muscles (see Chapter 7). Many types of peripheral neurons don’t undergo regular replacement in normal functioning. They are therefore some of the oldest cells in your body. Unfortunately, they’re not regenerated when they die from injury, so some kinds of nerve damage are permanent. Because they’re not replaced when they die, the number of such nerve cells declines throughout life.
- Ova: A woman has all the eggs she’s ever going to have in her ovaries at birth. In most women, that’s about half a million more than they’ll ever need. Most eggs die before puberty. Only a few mature and participate in the monthly events of the ovarian (menstrual) cycle. And only a very, very few go on to participate in the events of reproduction described in Chapter 14.
 Seeing what your body does automatically every day
Seeing what your body does automatically every day Finding out what goes on inside of every cell
Finding out what goes on inside of every cell Discovering the importance of homeostasis
Discovering the importance of homeostasis Building and maintaining your parts
Building and maintaining your parts Hetero means “other,” and tropho means “nourishment.” A heterotroph gets its nourishment from others, as opposed to an autotroph, which makes its own nourishment, as a plant does.
Hetero means “other,” and tropho means “nourishment.” A heterotroph gets its nourishment from others, as opposed to an autotroph, which makes its own nourishment, as a plant does. To keep the meanings of anabolic and catabolic clear in your mind, associate the word catabolic with the word catastrophic to remember that catabolic reactions break down products. Then you’ll know that anabolic reactions create products.
To keep the meanings of anabolic and catabolic clear in your mind, associate the word catabolic with the word catastrophic to remember that catabolic reactions break down products. Then you’ll know that anabolic reactions create products. So, nutrients are catabolized (broken down), ATP is formed (anabolized), and when needed, ATP is catabolized (for energy). This principle of linked anabolic and catabolic reactions is one of the cornerstones of human physiology and is required to maintain life. Cellular metabolism also makes waste products that must be removed (exported) from the cell and ultimately from the body.
So, nutrients are catabolized (broken down), ATP is formed (anabolized), and when needed, ATP is catabolized (for energy). This principle of linked anabolic and catabolic reactions is one of the cornerstones of human physiology and is required to maintain life. Cellular metabolism also makes waste products that must be removed (exported) from the cell and ultimately from the body.