Biogeochemical Cycles
Biogeochemical cycles are the backbone of Earth system science and are the major focus of this book. In this part, we will use the background provided in Part One, along with the information on the major reservoirs provided in Part Two to tell a cohesive story about five biogeochemical cycles: those of carbon ( Chapter 11), nitrogen ( Chapter 12), sulfur ( Chapter 13), phosphorus ( Chapter 14), and the trace metals ( Chapter 15). The goal of each one of these chapters is to provide an overview of the chemistry and isotopes of the element, describe the major reservoirs where the element resides and in what forms, provide estimates of the fluxes in and out of these reservoirs and the chemical transformations that accompany them, and finally to explain how the cycle has been altered by human activities. There is another element which is absolutely necessary for life on Earth that is not covered directly in this part: oxygen. As will be seen in the chapters that follow, oxygen is a part of other elemental cycles and often plays the part of an acid-maker and oxidizing agent. We have devoted an entire chapter to these processes, Chapter 16, which appears in the final part due to its integrative nature. What follows is a brief overview of the importance of each cycle, and a presentation of the highlights of each of these five chapters.
Carbon
The significance of the carbon cycle has relatively recently become appreciated by scientists and non-scientists alike, due to the buildup of carbon dioxide in the atmosphere. Out of all of the cycles we present here, it is perhaps the easiest one to use as an example of how humans have caused a change in an Earth system. Since fossil fuels are predominantly carbon, and are released as carbon dioxide when burned, we have essentially removed carbon from one reservoir (the lithosphere) and placed it directly into another (the atmosphere). Measurements of the concentration of CO2 have shown an increase in CO2 by about 15% since the measurements started in the 1950s and 30% since pre-industrial times. These findings are significant because CO2 is radiatively active in the atmosphere (it is a greenhouse gas). The more permanent uptake of CO2 is by the oceans. Since carbon is the major element in living things, there is a biospheric uptake by vegetation on the continents when they biochemically fix CO2 into organic matter, but this carbon returns to the atmosphere during plant respiration. There are other reduced forms of carbon in the atmosphere, such as methane (CH4) which also behave as greenhouse gases. A model of the carbon cycle is presented in this chapter. The data on the various fluxes and transformations were compiled using the 13C and 14C isotopes as tracers.
Nitrogen
Although carbon is the principal element in living organisms, the availability of nitrogen is often the limiting factor in plant growth. Thus, the cycle of nitrogen is intimately connected with the biosphere. Most of the biological interaction involves nitrogen fixation, which is any removal of N2 from the atmosphere to form any other nitrogen compound. In plants these compounds are usually ammonia or amino acids. Since the human diet depends so heavily on agriculture, we have added nitrogen to soils in the form of fertilizer in order to increase crop yields. Another way humans have altered the nitrogen cycle is by burning fossil fuels, which releases nitrogen oxides into the atmosphere. Atmospheric nitrogen in the form of oxides are a source of pollution, and a significant form of photochemical smog. These compounds play roles in both the formation and destruction of ozone in the troposphere and stratosphere, and can produce nitric acid after reaction with water. The acid rain that is produced can cause acidification of soils and bodies of water if the underlying bedrock lacks alkaline species. Changes in the nitrogen cycle therefore result in chemical conditions that directly affect public health and environmental welfare.
Sulfur
A great deal of attention has been paid to sulfur in the last decade or so because of its ubiquity in atmospheric particles which reflect sunlight back to space and affect the optical properties of clouds. These particles arise when SO2(g) reacts with (a) water, to form sulfuric acid, H2SO4, or (b) ammonia, NH3, to form ammonium sulfate, (NH4)2SO4, or ammonium bisulfate, NH4HSO4. The anthropogenic source of SO2 is generally from fossil fuel burning, whereas the major natural source is dimethyl sulfide (DMS), (CH3)2S, released from the oceans due to the activity of phytoplankton. The DMS oxidizes to SO2 once it enters the atmosphere and eventually forms sulfuric acid, ammonium sulfate, or ammonium bisulfate, just as in the anthropogenic case. Worldwide, sulfates are the dominant constituent in fine (< 1.0 µm diameter) particles, and are thought to exert a radiative effect on Earth that is similar in size to that of the CO2 greenhouse effect, although in the opposite direction (cooling rather than warming). Changes in the sulfur cycle therefore affect climate. The sulfuric acid that is produced in high concentrations in areas with large amounts of coal burning can acidify soils and lakes in the same way that nitric acid does. Sulfate is rarely a limiting nutrient and is very abundant in seawater. It is a very important oxidizing agent in anaerobic systems.
Phosphorus
Phosphorus is unusual in its chemistry compared to the other elements discussed here in that it exists in the environment almost completely in the P(V) oxidation state of phosphate,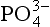 . Therefore, except in very small amounts occurring in atmospheric particles, the atmosphere is essentially not a reservoir for phosphorus and does not participate in its biogeochemical cycling. However, phosphorus is an extremely important constituent in all living things, forming the backbone of DNA, deoxyribonucleic acid. It is also the main source of cellular energy, in the form of ATP, adenosine triphosphate. The natural cycling of phosphorus, therefore, is completely intertwined with biospheric interaction. In the ocean, phosphorus in surface waters is rapidly taken up by biota which sink to lower depths and decompose. On land, phosphorus enters the soil through the decay of dead organic material. As mentioned in Chapter 8, the soil is eventually transported by rivers, and eventually lays down in a marine sediment. The phosphorus present in solution can participate as a bionutrient anywhere along this path. There has been a human disruption of the phosphorus cycle through the use of detergents and fertilizers, which cause overgrowth of organisms in natural waters, thus altering the ecosystem. There are theories that phosphorus, through its action as a nutrient, can effect the cycling of other elements, especially carbon. This could have important climate ramifications over geologic time periods.
. Therefore, except in very small amounts occurring in atmospheric particles, the atmosphere is essentially not a reservoir for phosphorus and does not participate in its biogeochemical cycling. However, phosphorus is an extremely important constituent in all living things, forming the backbone of DNA, deoxyribonucleic acid. It is also the main source of cellular energy, in the form of ATP, adenosine triphosphate. The natural cycling of phosphorus, therefore, is completely intertwined with biospheric interaction. In the ocean, phosphorus in surface waters is rapidly taken up by biota which sink to lower depths and decompose. On land, phosphorus enters the soil through the decay of dead organic material. As mentioned in Chapter 8, the soil is eventually transported by rivers, and eventually lays down in a marine sediment. The phosphorus present in solution can participate as a bionutrient anywhere along this path. There has been a human disruption of the phosphorus cycle through the use of detergents and fertilizers, which cause overgrowth of organisms in natural waters, thus altering the ecosystem. There are theories that phosphorus, through its action as a nutrient, can effect the cycling of other elements, especially carbon. This could have important climate ramifications over geologic time periods.
Trace Metals
In contrast with phosphorus, most metals can exist in a variety of oxidation states and physical forms, which makes them participants in all of the geospheres. However, because metals are generally trace elements for biota, most of the metal cycles are not significantly altered by biological interaction, but rather may affect the growth of organisms by acting as nutrients or poisons. Like the other cycles, we can identify the major ways that metals are mobilized into the environment. The major reservoir for all metals is in the lithosphere, so rock weathering and volcanic action are the main sources of natural metal cycling. From this starting point, metal ions enter the hydrosphere or the atmosphere, and are transformed depending on the conditions. The anthropogenic input of metals into the environment has received a great deal of attention in the last several decades because of the toxicity of the heavy metals. Mining, and subsequent use and disposal of metals into the atmosphere (e.g., from fly ash due to coal burning), the pedosphere (e.g., by burying metal ions), and the hydrosphere (e.g., by leaching of metal ions out of soils and sediments), have caused metals to enter the food chain. Probably the most well-known source of increased human consumption of metals is through fish and seafood, because some species accumulate the metals dissolved in water into their tissues.