Anomalies of the Penis and Urethra
Jack S. Elder
Hypospadias
Hypospadias is a urethral opening on the ventral surface of the penile shaft affecting 1 in 250 male newborns. Typically an isolated defect, its incidence is increased in many males with chromosomal abnormalities, anorectal malformation, and congenital heart disease. Usually, there is incomplete development of the prepuce, called a dorsal hood, in which the foreskin is on the sides and dorsal aspect of the penile shaft and deficient or absent ventrally. Some males with hypospadias, particularly those with proximal hypospadias, have chordee, in which there is ventral penile curvature during erection. The incidence of hypospadias appears to be increasing, possibly because of in utero exposure to estrogenic or antiandrogenic endocrine-disrupting chemicals (e.g., polychlorobiphenyls, phytoestrogens).
Clinical Manifestations
Hypospadias is classified according to the position of the urethral meatus after taking into account whether chordee is present (Fig. 559.1 ). The deformity is described as glanular (on the glans penis), coronal, subcoronal, midpenile, penoscrotal, scrotal, or perineal. Approximately 65% of cases are distal, 25% are subcoronal or midpenile, and 10% are proximal. In the most severe cases, the scrotum is bifid and sometimes there is moderate penoscrotal transposition. As many as 10% of affected males have a megameatal variant of hypospadias in which the foreskin is developed normally (megameatus intact prepuce variant) and there is either glanular or subcoronal hypospadias with a “fish mouth” meatus. These cases might not be diagnosed until after a circumcision is performed.
Approximately 10% of males with hypospadias have an undescended testis; an inguinal hernia(s) also are common. In the newborn, the differential diagnosis of midpenile or proximal hypospadias associated with an undescended testis should include forms of a disorder of sex development, particularly mixed gonadal dysgenesis, partial androgen insensitivity, true hermaphroditism, and congenital adrenal hyperplasia in a female (see Chapter 594 ). In the latter condition, neither gonad is palpable. A karyotype should be obtained in patients with midpenile or proximal hypospadias and cryptorchidism (see Chapter 606 ). In males with proximal hypospadias, a voiding cystourethrogram should be considered because 5–10% of these children have a dilated prostatic utricle, which is a remnant of the müllerian system (see Chapter 569 ). The incidence of upper urinary tract abnormalities is low unless there are abnormalities of other organ systems.
Complications of untreated hypospadias include deformity of the urinary stream, typically ventral deflection or severe splaying; sexual dysfunction secondary to penile curvature; infertility if the urethral meatus is proximal; meatal stenosis (congenital), which is uncommon; and cosmetic appearance. The goal of hypospadias surgery is to correct the functional and cosmetic deformities. Whereas hypospadias repair is recommended for all males with midpenile and proximal hypospadias, some males with distal hypospadias have no functional abnormality and do not need surgical correction.
Treatment
Management begins in the newborn period. Circumcision should be avoided because the foreskin often is used in the repair in most cases. The ideal age for repair in a healthy infant is 6-12 mo because the risk of general anesthesia at this age is similar to older children; penile growth over the next several years is slow; the child does not remember the surgical procedure; and postoperative analgesic needs are less than in older children. With the exception of proximal hypospadias, virtually all cases are repaired in a single operation on an ambulatory basis. The most common repair involves tubularization of the urethral plate distal to the urethral meatus, with coverage by a vascularized flap from the foreskin, termed a tubularized incised plate repair. Proximal cases might require a 2-stage repair. The complication rate is low: 5% for distal hypospadias, 10% for midpenile hypospadias, and 40% for proximal hypospadias. The most common complications include urethrocutaneous fistula and meatal stenosis. Other complications include a deformed urinary stream, persistent penile curvature, and dehiscence of the hypospadias repair. Treatment of these complications generally is straightforward. In complex cases, a buccal mucosa graft from the mouth is used to create urethral mucosa. Repair of hypospadias is a technically demanding operation and should be performed by a surgeon with specialty training in pediatric urology and extensive experience.
Chordee Without Hypospadias
In some males, there is mild or moderate ventral penile curvature (chordee) and incomplete development of the foreskin (dorsal hood), but the urethral meatus is at the tip of the glans (Fig. 559.2 ). In most of these males, the urethra is normal but there is insufficient ventral penile skin or prominent, inelastic ventral bands of dartos fascia that prevent a straight erection. In some cases, the urethra is hypoplastic, and a formal urethroplasty is necessary for repair. The only sign of this anomaly in the neonate may be the hooded foreskin, and delayed repair under general anesthesia after 6 mo of age is recommended. Lateral penile curvature usually is caused by overgrowth or hypoplasia of a corporal (erectile) body and usually is congenital. Surgical repair is recommended at age 6-12 mo.

Phimosis and Paraphimosis
Phimosis refers to the inability to retract the prepuce. At birth, phimosis is physiologic. Over time, the adhesions between the prepuce and glans lyse and the distal phimotic ring loosens. In 80% of uncircumcised males, the prepuce becomes retractable by 3 yr of age. Accumulation of epithelial debris under the infant's prepuce is physiologic and does not mandate circumcision. In older males, phimosis may be physiologic, or may be pathologic from lichen sclerosus (balanitis xerotica obliterans) at the tip of the foreskin (Fig. 559.3A ) and can affect the meatus also (see Fig. 559.3B ). The prepuce might have been retracted forcefully on one or two occasions in the past, which can result in a cicatricial scar that prevents subsequent retraction of the foreskin. In males with persistent physiologic or pathologic phimosis, application of corticosteroid ointment to the tip of the foreskin two times daily for 1 mo loosens the phimotic ring in two thirds of cases. If there is ballooning of the foreskin during voiding or phimosis beyond 10 yr of age and topical corticosteroid therapy is ineffective, circumcision is recommended.
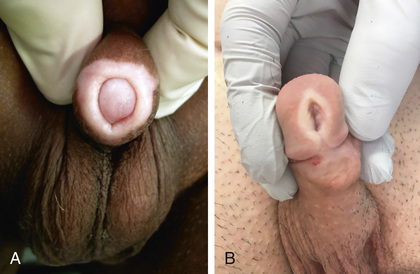
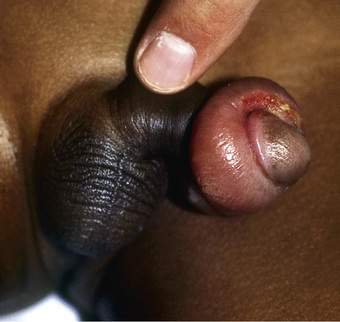
Paraphimosis occurs when the foreskin is retracted proximal to the coronal sulcus and the prepuce cannot be pulled back over the glans (Fig. 559.4 ). Painful venous stasis in the retracted foreskin results, with edema leading to severe pain and inability to reduce the foreskin (pull it back over the glans). Treatment includes lubricating the foreskin and glans and then simultaneously compressing the glans and placing distal traction on the foreskin to try to push the phimotic ring past the coronal sulcus. Topical application of granulated sugar has been reported to aid in reduction of edema by creation of an osmotic gradient, facilitating reduction of paraphimosis. In addition, injection of hyaluronidase into the edematous skin has been reported to result in immediate reduction in swelling. In rare cases, emergency circumcision under general anesthesia is necessary.
Circumcision
In the United States, circumcision usually is performed for cultural reasons. In 2012, a multidisciplinary task force of the American Academy of Pediatrics stated that evidence indicates that the health benefits of newborn male circumcision outweigh the risks and that the procedure's benefits justify access to this procedure for families who choose it. Specific benefits identified included prevention of urinary tract infections (UTIs) and penile cancer and reducing the risk and transmission of some sexually transmitted infections, including HIV. The American College of Obstetricians and Gynecologists endorsed this policy statement. By contrast, European medical professional groups have been less likely to endorse this practice.
When performing a neonatal circumcision, local analgesia, such as a dorsal or penile ring block or application of EMLA (eutectic mixture of local anesthetics) cream (lidocaine 2.5% and prilocaine 2.5%) is recommended.
UTIs are 10-15 times more common in uncircumcised infant males than in circumcised infants, with the urinary pathogens arising from bacteria that colonize the space between the prepuce and glans. The risk of febrile UTI (see Chapter 553 ) is highest between birth and 6 mo, but there is an increased risk of UTI until at least 5 yr of age. Many recommend circumcision in infants who are predisposed to UTI, such as those with congenital hydronephrosis and vesicoureteral reflux. Circumcision reduces the risk of sexually transmitted infections in adults (see Chapter 146 ), in particular HIV (see Chapter 302 ). There have been only a handful of reports of adult males who were circumcised at birth and subsequently acquired penile carcinoma, but in Scandinavian countries, where few males are circumcised and hygiene is good, the incidence of penile cancer is low.
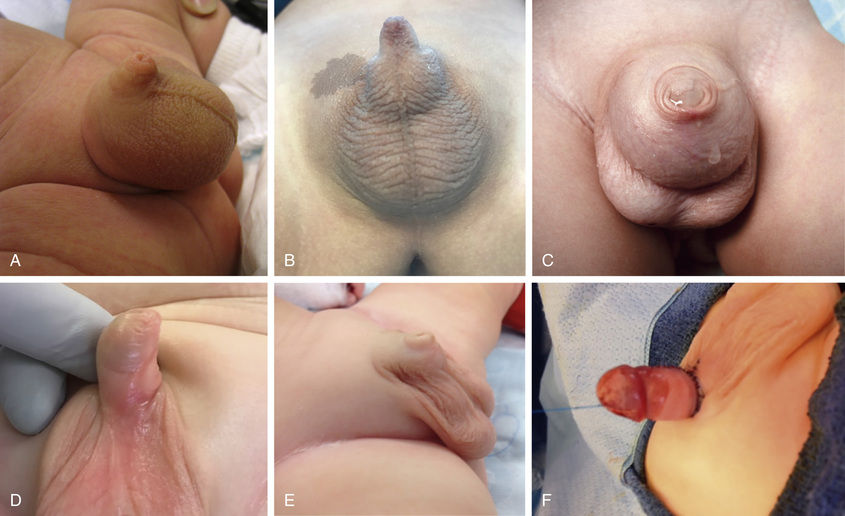
Early and late complications after neonatal circumcision include bleeding, wound infection, meatal stenosis, secondary phimosis, removal of insufficient foreskin, and fibrous penile adhesions (skin bridge; Fig. 559.5 ); 0.2–3.0% of patients undergo a subsequent operative procedure. Males with a large hydrocele or hernia are at particular risk for secondary phimosis because the scrotal swelling tends to displace the penile shaft skin over the glans. Serious complications of newborn circumcision include sepsis, amputation of the distal part of the glans, removal of an excessive amount of foreskin, and urethrocutaneous fistula. Circumcision should not be performed in neonates with hypospadias, chordee without hypospadias, or a dorsal hood deformity (relative contraindication) or in those with a small penis (see Fig. 559.6 ). In males with a “wandering raphe” (see Fig. 559.6 ), in which the median raphe deviates to one side, there may be underlying penile torsion or hypospadias, and evaluation by a pediatric urologist is suggested before performing a circumcision.
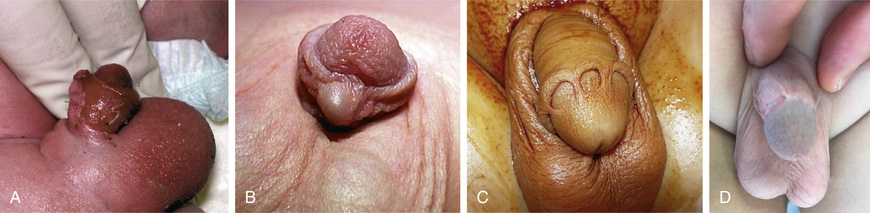
Penile Torsion
Penile torsion, a rotational defect of the penile shaft, usually occurs in a counterclockwise direction, usually to the left side (see Fig. 559.6 ). In most cases, penile development is normal, and the condition is unrecognized until circumcision is performed or the foreskin is retractable. In many cases, the midline raphe of the penile shaft is deviated. Penile torsion also occurs in some males with hypospadias. The defect has primarily cosmetic significance, and correction is unnecessary if the rotation is < 60 degrees from the midline. The severity of penile torsion may lessen during infancy.
Inconspicuous Penis
The term inconspicuous penis refers to a penis that appears to be small. A webbed penis is a condition in which the scrotal skin extends onto the ventral penile shaft. This deformity represents an abnormality of the attachment between the penis and scrotum. Although the deformity might appear mild, if a routine circumcision is performed, the penis can retract into the scrotum, resulting in secondary phimosis (trapped penis). The concealed (hidden or buried) penis is a normally developed penis that is camouflaged by the suprapubic fat pad (Fig. 559.7 ). This anomaly may be congenital, iatrogenic after circumcision, or a result of obesity. Surgical correction is indicated for cosmetic reasons or if there is a functional abnormality with a splayed stream.
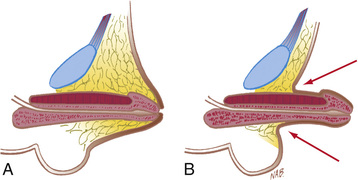
A trapped penis is an acquired form of inconspicuous penis and refers to a phallus that becomes embedded in the suprapubic fat pad after circumcision (Fig. 559.8 ). This deformity can occur after neonatal circumcision in an infant who has significant scrotal swelling from a large hydrocele or inguinal hernia or after routine circumcision in an infant with a webbed penis. This complication can predispose to UTIs and can cause urinary retention. Initial treatment of a trapped penis should include topical corticosteroid cream, which often loosens the phimotic ring. In some cases, secondary repair is necessary at 6-9 mo.

Micropenis
Micropenis is defined as a normally formed penis that is at least 2.5 SD below the mean in size (Fig. 559.9 ). Typically, the ratio of the length of the penile shaft to its circumference is normal. The pertinent measurement is the stretched penile length , which is measured by stretching the penis and measuring the distance from the penile base under the pubic symphysis to the tip of the glans. The mean length of the term newborn penis is 3.5 ± 0.7 cm and the diameter is 1.1 ± 0.2 cm. The diagnosis of micropenis in a male newborn is if the stretched length is < 1.9 cm.

Micropenis usually results from a hormonal abnormality that occurs after 14 wk of gestation. Common causes include hypogonadotropic hypogonadism, hypergonadotropic hypogonadism (primary testicular failure), and idiopathic micropenis. If growth hormone deficiency also is present, neonatal hypoglycemia can occur. The most common cause of micropenis is failure of the hypothalamus to produce an adequate amount of gonadotropin-releasing hormone, as typically occurs in Kallmann syndrome (see Chapter 601 ), Prader-Willi syndrome (see Chapter 98 ), and Lawrence-Moon-Bardet-Biedl syndrome. In some cases, there is growth hormone deficiency. Primary testicular failure can result from gonadal dysgenesis or rudimentary testes syndrome and also occurs in Robinow syndrome (characterized by hypoplastic genitalia, shortening of the forearms, frontal bossing, hypertelorism, wide palpebral fissures, short broad nose, long philtrum, small chin, brachydactyly, and a normal karyotype).
A pediatric endocrinologist, geneticist, and pediatric urologist should examine all children with these syndromes, with participation by medical ethics. Evaluation includes a karyotype, assessment of anterior pituitary function and testicular function, and MRI to determine the anatomic integrity of the hypothalamus and the anterior pituitary gland as well as the midline structure of the brain. One of the difficult questions is whether androgen therapy is essential during childhood, because androgenic stimulation of penile growth in a prepubertal male can limit the growth potential of the penis in puberty. Studies of small groups of men with micropenis suggest that many, although not all, have satisfactory sexual function. Consequently, a decision for gender reassignment is made infrequently.
Priapism
Priapism is a persistent penile erection at least 4 hr in duration that continues beyond, or is unrelated to, sexual stimulation. Typically, only the corpora cavernosa is affected. There are three subtypes:
- ◆ Ischemic (venoocclusive, low-flow) priapism is characterized by little or no cavernous blood flow, and cavernous blood gases are hypoxic, hypercapnic, and acidotic. The corpora are rigid and tender to palpation.
- ◆ Nonischemic (arterial, high-flow) priapism is caused by unregulated cavernous arterial inflow. Typically, the penis is neither fully rigid nor painful. There is often a history of antecedent trauma resulting in a cavernous artery–corpora cavernosa fistula.
- ◆ Stuttering (intermittent) priapism is a recurrent form of ischemic priapism with painful erections with intervening periods of detumescence.
The most common cause of priapism in children is sickle cell disease, which is characterized by a predominance of sickle cell hemoglobin (see Chapter 489.1 ). As many as 27.5% of children with sickle cell disease develop priapism. The priapism is generally related to a low-flow state, secondary to sickling of red blood cells within the sinusoids of the corpora cavernosa during normal erection, resulting in venous stasis. This situation results in decreased local oxygen tension and pH, which potentiates further stasis and sickling. Priapism typically occurs during sleep, when mild hypoventilatory acidosis depresses oxygen tension and pH in the corpora. There is typically significant corporal engorgement with sparing of the glans penis. If the spongiosum is involved, voiding may be impaired. Evaluation includes a complete blood count and serum chemistry. If the sickle cell status is unknown, hemoglobin electrophoresis should be performed. In some cases, corporal aspiration is performed to distinguish between a high-flow and low-flow state. Other causes of low-flow priapism include sildenafil ingestion and leukemia.
In priapism secondary to sickle cell disease, medical therapy includes exchange transfusion, intravenous hydration, alkalinization, pain management with morphine, and oxygen. The American Urological Association guideline on priapism also recommends concurrent intracavernous treatment beginning with corporal aspiration and irrigation with a sympathomimetic agent, such as phenylephrine. If priapism has been present for > 48 hr, ischemia and acidosis impair the intracavernous smooth muscle response to sympathomimetics. If irrigation and medical therapy are unsuccessful, a corporoglanular shunt should be considered. For stuttering priapism, administration of an oral α-adrenergic agent (pseudoephedrine) once or twice daily is first-line therapy. If this treatment is unsuccessful, an oral β-agonist (terbutaline) is recommended; a gonadotropin-releasing hormone analog plus flutamide is recommended as third-line therapy. Long-term follow-up of adults treated for sickle cell disease as children shows that satisfactory erectile function is inversely related to the patient's age at the onset of priapism and duration of priapism.
Nonischemic (high-flow) priapism most commonly follows perineal trauma, such as a straddle injury, that results in laceration of the cavernous artery. Typically, the aspirated blood is bright red, and the aspirate is similar to arterial blood. Color Doppler ultrasonography often demonstrates the fistula. The priapism can spontaneously resolve. If it does not, angiographic embolization is indicated.
Other Penile Anomalies
Agenesis of the penis affects approximately 1 in 10 million males. The karyotype is almost always 46, XY, and the usual appearance is that of a well-developed scrotum with descended testes and an absent penile shaft. Upper urinary tract abnormalities are common. In most cases, gender reassignment is recommended in the newborn period. Diphallia ranges from a small accessory penis to complete duplication.
Meatal Stenosis
Meatal stenosis is a condition that almost always is acquired and occurs after neonatal circumcision. Most probably, it probably results from inflammation of the denuded glans and is difficult to prevent. If the meatus is pinpoint, males void with a forceful, fine stream that goes a great distance. These males can experience dysuria, frequency, hematuria, or a combination of these conditions, typically at age 3-8 yr. UTI is uncommon. Other males have dorsal deflection of the urinary stream. Although the meatus may be small, hydronephrosis or voiding difficulty is extremely rare unless there is associated balanitis xerotica obliterans (see Fig. 559.3 ; chronic dermatitis of unknown etiology, generally involving the glans and prepuce, occasionally extending into the urethra). Treatment is meatoplasty, in which the urethral meatus is opened surgically; this procedure can be performed either under anesthesia as an outpatient or in the office using local anesthesia (EMLA cream) with or without sedation. Routine cystoscopy is unnecessary.
Other Male Urethral Anomalies
Parameatal urethral cyst manifests as an asymptomatic small cyst on one side of the urethral meatus. Treatment is excision under anesthesia. Congenital urethral fistula is a rare deformity in which a fistula is present from the penile urethra. It usually is an isolated abnormality. Treatment is fistula closure. Megalourethra is a large urethra that usually is associated with abnormal development of the corpus spongiosum. This condition is most commonly associated with prune-belly syndrome (see Chapter 555 ). Urethral duplication is a rare condition in which the two urethral channels lie in the same sagittal plane. There are many variations with complete and incomplete urethral duplication. These males often have a double stream. Most commonly, the dorsal urethra is small and the ventral urethra is of normal caliber. Treatment involves excision of the small urethra. Urethral hypoplasia is a rare condition in which the entire male urethra is extremely small but patent. In some cases, a temporary cutaneous vesicostomy is necessary for satisfactory urinary drainage. Either gradual enlargement of the urethra or major urethroplasty is necessary. Urethral atresia refers to maldevelopment of the urethra and nearly always is fatal unless the urachus remains patent throughout gestation.
Urethral Prolapse (Female)
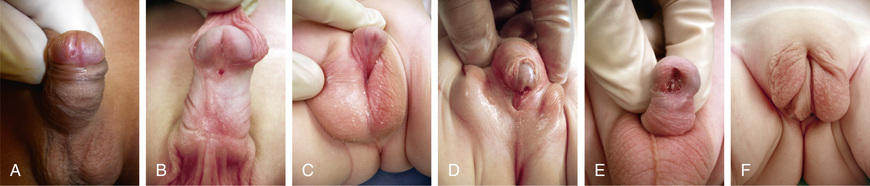
Urethral prolapse occurs predominantly in black females 1-9 yr of age. The most common signs are bloody spotting on the underwear or diaper, although dysuria or perineal discomfort also can occur (Fig. 559.10 ). An inexperienced examiner can mistake the finding for sexual abuse. Initial therapy consists of application of estrogen cream 2-3 times daily for 3-4 wk and sitz baths. Manual lysis is recommended for females that fail medical therapy and is curative. In some cases, this can be performed in the office under local anesthesia.

Other Female Urethral Lesions
Paraurethral cyst results from retained secretions in the Skene glands secondary to ductal obstruction (Fig. 559.11 ). These lesions are present at birth, and most regress in size during the first 4-8 wk, although occasionally incision and drainage is necessary. A prolapsed ectopic ureterocele appears as a cystic mass protruding from the urethra and is a presenting symptom in 10% of females with a ureterocele, which is a cystic swelling of the terminal ureter (Fig. 559.12 ). Ultrasonography should be performed to visualize the upper urinary tracts to confirm the diagnosis. Usually, either the ureterocele is incised or an upper urinary tract reconstructive procedure is necessary.

