25
Psychiatric Disorders
Kristin S. Raj, MD
Nolan Williams, MD
Charles DeBattista, DMH, MD
The fifth edition of the American Psychiatric Association’s Diagnostic and Statistical Manual (DSM-5) is the common language that clinicians use for psychiatric conditions. It utilizes specific criteria with which to objectively assess symptoms for use in clinical diagnosis and communication.
COMMON PSYCHIATRIC DISORDERS
ADJUSTMENT DISORDERS
ESSENTIALS OF DIAGNOSIS

 Anxiety or depression in reaction to an identifiable stress, though out of proportion to the severity of the stressor.
Anxiety or depression in reaction to an identifiable stress, though out of proportion to the severity of the stressor.
 Symptoms are not at the severity of a major depressive episode or with the chronicity of generalized anxiety disorder (GAD).
Symptoms are not at the severity of a major depressive episode or with the chronicity of generalized anxiety disorder (GAD).
 General Considerations
General Considerations
An individual experiences stress when adaptive capacity is overwhelmed by events. The event may be an insignificant one when objectively considered, and even favorable changes (eg, promotion and transfer) requiring adaptive behavior can produce stress. For everyone, stress is subjectively defined, and the response to stress is a function of each person’s personality and physiologic endowment.
Opinion differs about what events are most apt to produce stress reactions. The causes of stress are different at different ages—eg, in young adulthood, the sources of stress are found in the marriage or parent-child relationship, the employment relationship, and the struggle to achieve financial stability; in the middle years, the focus shifts to changing spousal relationships, problems with aging parents, and problems associated with having young adult offspring who themselves are encountering stressful situations; in old age, the principal concerns are apt to be retirement, loss of physical and mental capacity, major personal losses, and thoughts of death.
 Clinical Findings
Clinical Findings
An individual may react to stress by becoming anxious or depressed, by developing a physical symptom, by running away, drinking alcohol, overeating, starting an affair, or in limitless other ways. Common subjective responses are anxiety, sadness, fear, rage, guilt, and shame. Acute and reactivated stress may be manifested by restlessness, irritability, fatigue, increased startle reaction, and a feeling of tension. Inability to concentrate, sleep disturbances (insomnia, bad dreams), and somatic preoccupations sometimes lead to self-medication, most commonly with alcohol or other central nervous system depressants. Emotional and behavioral distressing symptomatology in response to stress is called adjustment disorder, with the major symptom specified (eg, “adjustment disorder with depressed mood”). Even with an identifiable stressor, if the patient meets syndromal criteria for another disorder such as major depression, then the convention would be to diagnose a major depression and not an adjustment disorder with depressed mood.
 Differential Diagnosis
Differential Diagnosis
Adjustment disorders are distinguished from anxiety disorders, mood disorders, bereavement, other stress disorders such as posttraumatic stress disorder (PTSD), and personality disorders exacerbated by stress and from somatic disorders with psychic overlay. Unlike many other psychiatric disorders, such as bipolar disorder or schizophrenia, adjustment disorders are wholly situational and usually resolve when the stressor resolves or the individual effectively adapts to the situation. Adjustment disorders may have symptoms that overlap with other disorders, such as anxiety symptoms, but they occur in reaction to an identifiable life stressor such as a difficult work situation or romantic breakup. An adjustment disorder that persists and worsens can potentially evolve into another psychiatric disorder such as major depression or GAD. However, that is not the case for most patients. Patients with adjustment disorders have marked distress after a stressor and significant impairment in social or occupational functioning but not to the degree experienced by patients with a more severe disorder such as major depressive disorder or PTSD. By definition, an adjustment disorder occurs within 3 months of an identifiable stressor.
 Treatment
Treatment
A. Behavioral
Stress reduction techniques include immediate symptom reduction (eg, rebreathing in a bag for hyperventilation) or early recognition and removal from a stress source before full-blown symptoms appear. It is often helpful for the patient to keep a daily log of stress precipitators, responses, and alleviators. Relaxation, mindfulness-based stress reduction, and exercise techniques are also helpful in improving the reaction to stressful events.
B. Social
The stress reactions of life crisis problems are a function of psychosocial upheaval. While it is not easy for the patient to make necessary changes (or they would have been made long ago), it is important for the clinician to establish the framework of the problem, since the patient’s denial system may obscure the issues. Clarifying the problem in the patient’s psychosocial context allows the patient to begin viewing it within the proper frame and facilitates the difficult decisions the patient eventually must make (eg, change of job).
C. Psychological
Prolonged in-depth psychotherapy is seldom necessary in cases of isolated stress response or adjustment disorder. Supportive psychotherapy (see above) with an emphasis on strengthening of existing coping mechanisms is a helpful approach so that time and the patient’s own resiliency can restore the previous level of function. In addition, cognitive behavioral therapy has long been established to treat acute stress and facilitate recovery in patients with an adjustment disorder.
D. Pharmacologic
Judicious use of sedatives (eg, lorazepam, 0.5–1 mg two or three times daily orally) for a limited time and as part of an overall treatment plan can provide relief from acute anxiety symptoms. Problems arise when the situation becomes chronic through inappropriate treatment or when the treatment approach supports the development of chronicity. There are occasions where the short-term use of selective serotonin reuptake inhibitors (SSRIs) targeting dysphoria and anxiety may be useful.
 Prognosis
Prognosis
Return to satisfactory function after a short period is part of the clinical picture of this syndrome. Resolution may be delayed if others’ responses to the patient’s difficulties are thoughtlessly harmful or if the secondary gains outweigh the advantages of recovery. The longer the symptoms persist, the worse the prognosis. There is also evidence that stress-related disorders are associated with increased risk of autoimmune disease, although this mechanism has yet to be elucidated.
Song H et al. Association of stress-related disorders with subsequent autoimmune disease. JAMA. 2018 Jun 19;319(23):2388–400. [PMID: 29922828]
TRAUMA & STRESSOR-RELATED DISORDERS
ESSENTIALS OF DIAGNOSIS

 Exposure to a traumatic or life-threatening event.
Exposure to a traumatic or life-threatening event.
 Flashbacks, intrusive images, and nightmares, often represent reexperiencing the event.
Flashbacks, intrusive images, and nightmares, often represent reexperiencing the event.
 Avoidance symptoms, including numbing, social withdrawal, and avoidance of stimuli associated with the event.
Avoidance symptoms, including numbing, social withdrawal, and avoidance of stimuli associated with the event.
 Increased vigilance, such as startle reactions and difficulty falling asleep.
Increased vigilance, such as startle reactions and difficulty falling asleep.
 Symptoms impair functioning.
Symptoms impair functioning.
 General Considerations
General Considerations
Posttraumatic stress disorder (PTSD) has been reclassified from an anxiety disorder to a trauma and stressor-related disorder in the DSM-5. PTSD is a syndrome characterized by “reexperiencing” a traumatic event (eg, sexual assault, severe burns, military combat) and decreased responsiveness and avoidance of current events associated with the trauma. The lifetime prevalence of PTSD among adult Americans has been estimated to be 6.8% with a point prevalence of 3.6% and with women having rates twice as high as men. Many individuals with PTSD (20–40%) have experienced other associated problems, including divorce, parenting problems, difficulties with the law, and substance abuse.
 Clinical Findings
Clinical Findings
The key to establishing the diagnosis of PTSD lies in the history of exposure to a perceived or actual life-threatening event, serious injury, or sexual violence. This can include serious medical illnesses, and the prevalence of PTSD is higher in people who have experienced serious illnesses such as cancer. The symptoms of PTSD include intrusive thoughts (eg, flashbacks, nightmares), avoidance (eg, withdrawal), negative thoughts and feelings, and increased reactivity. Patients with PTSD can experience physiologic hyperarousal, including startle reactions, illusions, overgeneralized associations, sleep problems, nightmares, dreams about the precipitating event, impulsivity, difficulties in concentration, and hyperalertness. The symptoms may be precipitated or exacerbated by events that are a reminder of the original traumatic event. Symptoms frequently arise after a long latency period (eg, child abuse can result in later-onset PTSD). DSM-5 includes the requirement that the symptoms persist for at least 1 month. In some individuals, the symptoms fade over months or years, and in others they may persist for a lifetime.
 Differential Diagnosis
Differential Diagnosis
In 75% of cases, PTSD occurs with comorbid depression or panic disorder, and there is considerable overlap in the symptom complexes of all three conditions. Acute stress disorder has many of the same symptoms as PTSD, but symptoms persist for only 3 days to a month after the trauma. The other major comorbidity is alcohol and substance abuse. The Primary Care-PTSD Screen and the PTSD Checklist are two useful screening instruments in primary care clinics or community settings with populations at risk for trauma exposure.
 Treatment
Treatment
A. Psychotherapy
Psychotherapy should be initiated as soon as possible after the traumatic event, and it should be brief (typically 8–12 sessions), once the individual is in a safe environment. Cognitive processing therapy, cognitive behavioral therapy, and prolonged exposure therapy have demonstrated the greatest evidence in improving symptoms of PTSD. Evidence also shows that these psychotherapies may be more beneficial than medications alone for PTSD. In these approaches, the individual confronts the traumatic situation and learns to view it with less reactivity. Posttraumatic stress syndromes respond to interventions that help patients integrate the event in an adaptive way with some sense of mastery in having survived the trauma. Partner relationship problems are a major area of concern, and it is important that the clinician have available a dependable referral source when marriage counseling is indicated.
Treatment of any comorbid substance abuse is an essential part of the recovery process for patients with PTSD. In patients with comorbid substance use disorders, there is evidence for better outcomes when substance abuse treatment is delivered alongside trauma-focused psychotherapy. Support groups and 12-step programs such as Alcoholics Anonymous are often very helpful.
Video telepsychiatry for psychotherapy or medication management allows for access to these resources that some patients, such as those in rural settings, may not otherwise have. There is similar efficacy in reduction of PTSD symptoms in women veterans with video teletherapy as with in-person therapy, and the practice of telepsychiatry is becoming more widely available.
B. Pharmacotherapy
SSRIs are helpful in ameliorating depression, panic attacks, sleep disruption, and startle responses in PTSD. Sertraline and paroxetine are approved by the US Food and Drug Administration (FDA) for this purpose, and the SSRIs are the only class of medications approved for the treatment of PTSD. They are, therefore, considered the pharmacotherapy of choice for PTSD. Early treatment of anxious arousal with beta-blockers (eg, propranolol, 80–160 mg orally daily) may lessen the peripheral symptoms of anxiety (eg, tremors, palpitations) but has not been shown to help prevent development of PTSD. Similarly, noradrenergic agents such as clonidine (titrated from 0.1 mg orally at bedtime to 0.2 mg three times a day) have been shown to help with the hyperarousal symptoms of PTSD. The alpha-adrenergic blocking agent prazosin (2–10 mg orally at bedtime) has mixed evidence for decreasing nightmares and improving quality of sleep in PTSD, with a recent negative randomized control trial. Benzodiazepines, such as clonazepam, are generally thought to be contraindicated in the treatment of PTSD. The risks of benzodiazepines, including addiction and disinhibition, are thought to outweigh the anxiolytic and sleep benefits in most patients. Trazodone (25–100 mg orally at bedtime) is commonly prescribed as a non–habit forming hypnotic agent. Second-generation antipsychotics have not demonstrated significant utility in the treatment of PTSD, but agents such as quetiapine 50–300 mg/day may have a limited role in treating agitation and sleep disturbance in PTSD patients. Work with novel agents such as MDMA (methylenedioxymethamphetamine; also called Ecstasy) have shown early promise in the treatment of PTSD and are under investigation.
 Prognosis
Prognosis
The sooner therapy is initiated after the trauma, the better the prognosis. A study published in 2018 comparing sertraline and prolonged exposure therapy for patients with PTSD demonstrated that patients who received their preferred treatment were more likely to be adherent, respond to treatment, and have lower self-reported PTSD, depression, and anxiety symptoms. Approximately half of patients with PTSD experience chronic symptoms. Prognosis is best in those with good premorbid psychiatric functioning. Individuals experiencing an acute stress disorder typically do better long-term than those experiencing a delayed posttraumatic disorder. Individuals who experience trauma resulting from a natural disaster (eg, earthquake or hurricane) tend to do better than those who experience a traumatic interpersonal encounter (eg, rape or combat).
Guideline Development Panel for the Treatment of PTSD in Adults, American Psychological Association. Summary of the clinical practice guideline for the treatment of posttraumatic stress disorder (PTSD) in adults. Am Psychol. 2019 Jul–Aug;74(5):596–607. [PMID: 31305099]
Merz J et al. Comparative efficacy and acceptability of pharmacological, psychotherapeutic, and combination treatments in adults with posttraumatic stress disorder: a network meta-analysis. JAMA Psychiatry. 2019;76(9):904–13. [PMID: 31188399]
Raskind MA et al. Trial of prazosin for post-traumatic stress disorder in military veterans. N Engl J Med. 2018 Feb 8;378(6):507–17. [PMID: 29414272]
Zoellner LA et al. Doubly randomized preference trial of prolonged exposure versus sertraline for treatment of PTSD. Am J Psychiatry. 2019 Apr 1;176(4):287–96. [PMID: 30336702]
ANXIETY DISORDERS
ESSENTIALS OF DIAGNOSIS

 Persistent excessive anxiety or chronic fear and associated behavioral disturbances.
Persistent excessive anxiety or chronic fear and associated behavioral disturbances.
 Somatic symptoms referable to the autonomic nervous system or to a specific organ system (eg, dyspnea, palpitations, paresthesias).
Somatic symptoms referable to the autonomic nervous system or to a specific organ system (eg, dyspnea, palpitations, paresthesias).
 Not limited to an adjustment disorder.
Not limited to an adjustment disorder.
 Not a result of physical disorders, other psychiatric conditions (eg, schizophrenia), or drug abuse (eg, cocaine).
Not a result of physical disorders, other psychiatric conditions (eg, schizophrenia), or drug abuse (eg, cocaine).
 General Considerations
General Considerations
Stress, fear, and anxiety all tend to be interactive. The principal components of anxiety are psychological (tension, fears, difficulty in concentration, apprehension) and somatic (tachycardia, hyperventilation, shortness of breath, palpitations, tremor, sweating). Sympathomimetic symptoms of anxiety are both a response to a central nervous system state and a reinforcement of further anxiety. Anxiety can become self-generating, since the symptoms reinforce the reaction, causing it to spiral. Additionally, avoidance of triggers of anxiety leads to reinforcement of the anxiety. The person continues to associate the trigger with anxiety and never relearns through experience that the trigger need not always result in fear, or that anxiety will naturally improve with prolonged exposure to an objectively neutral stressor.
Anxiety may be free-floating, resulting in acute anxiety attacks, occasionally becoming chronic. Lack of structure is frequently a contributing factor, as noted in those people who have “Sunday neuroses.” They do well during the week with a planned work schedule but cannot tolerate the unstructured weekend. Planned-time activities tend to bind anxiety, and many people have increased difficulties when this is lost, as in retirement.
 Clinical Findings
Clinical Findings
A. Generalized Anxiety Disorder
Anxiety disorders are the most prevalent psychiatric disorders. About 7% of women and 4% of men will meet criteria for GAD over a lifetime. GAD becomes chronic in many patients with over half of patients having the disorder for longer than 2 years. Anxiety disorder in the elderly is twice as common as dementia and four to six times more common than major depression, and it is associated with poorer quality of life and contributes to the onset of disability. The anxiety symptoms of apprehension, worry, irritability, difficulty in concentrating, insomnia, or somatic complaints are present more days than not for at least 6 months. Manifestations can include cardiac (eg, tachycardia, increased blood pressure), gastrointestinal (eg, increased acidity, nausea, epigastric pain), and neurologic (eg, headache, near-syncope) systems. The focus of the anxiety may be a number of everyday activities.
B. Panic Disorder
Panic attacks are recurrent, unpredictable episodes of intense surges of anxiety accompanied by marked physiologic manifestations. Agoraphobia, fear of being in places where escape is difficult, such as open spaces or public places where one cannot easily hide, may be present and may lead the individual to confine his or her life to home. Distressing symptoms and signs such as dyspnea, tachycardia, palpitations, dizziness, paresthesias, choking, smothering feelings, and nausea are associated with feelings of impending doom (alarm response). Although these symptoms may lead to overlap with some of the same bodily complaints found in the somatic symptom disorders, the key to the diagnosis of panic disorder is the psychic pain and suffering the individual expresses. Panic disorder is diagnosed when panic attacks are accompanied by a chronic fear of the recurrence of an attack or a maladaptive change in behavior to try to avoid potential triggers of the panic attack. Recurrent sleep panic attacks (not nightmares) occur in about 30% of panic disorders. Anticipatory anxiety develops in all these patients and further constricts their daily lives. Panic disorder tends to be familial, with onset usually under age 25; it affects 3–5% of the population, and the female-to-male ratio is 2:1. The premenstrual period is one of heightened vulnerability. Patients frequently undergo evaluations for emergent medical conditions (eg, heart attack or hypoglycemia), which are then ruled out before the correct diagnosis is made. Gastrointestinal symptoms (eg, stomach pain, heartburn, diarrhea, constipation, nausea and vomiting) are common, occurring in about one-third of cases. Myocardial infarction, pheochromocytoma, hyperthyroidism, and various recreational drug reactions can mimic panic disorder and should be considered in the differential diagnosis. Patients who have panic disorder can become demoralized, hypochondriacal, agoraphobic, and depressed. These individuals are at increased risk for major depression and suicide. Alcohol abuse (in about 20%) results from self-treatment and is frequently combined with dependence on sedatives. Some patients have atypical panic attacks associated with seizure-like symptoms that often include psychosensory phenomena (a history of stimulant abuse often emerges). About 25% of panic disorder patients also have obsessive-compulsive disorder (OCD).
C. Phobic Disorders
Simple phobias are fears of a specific object or situation (eg, spiders, height) that are out of proportion to the danger posed, and they tend to be chronic. Social phobias are global or specific; in the former, all social situations are poorly tolerated, while the latter group includes performance anxiety (eg, fear of public speaking). While patients with simple phobias such as fear of heights may function as long as they do not have to be in tall buildings or airplanes, a patient with agoraphobia may not be able to function vocationally or interpersonally.
 Treatment
Treatment
In all cases, underlying medical disorders must be ruled out (eg, cardiovascular, endocrine, respiratory, and neurologic disorders and substance-related syndromes, both intoxication and withdrawal states).
A. Pharmacologic
1. Generalized anxiety disorder—Antidepressants including the SSRIs and serotonin norepinephrine reuptake inhibitors (SNRIs) are first-line treatment and safe and effective in the long-term management of GAD. Some patients may find more noradrenergic SNRIs such as levomilnacipran too activating to tolerate. The antidepressants appear to be as effective as the benzodiazepines without the risks of tolerance or dependence. However, benzodiazepines take effect more quickly if not immediately, which can be beneficial in brief acute management (Table 25–1).
Table 25–1. Commonly used antianxiety and hypnotic agents (listed in alphabetical order within classes).
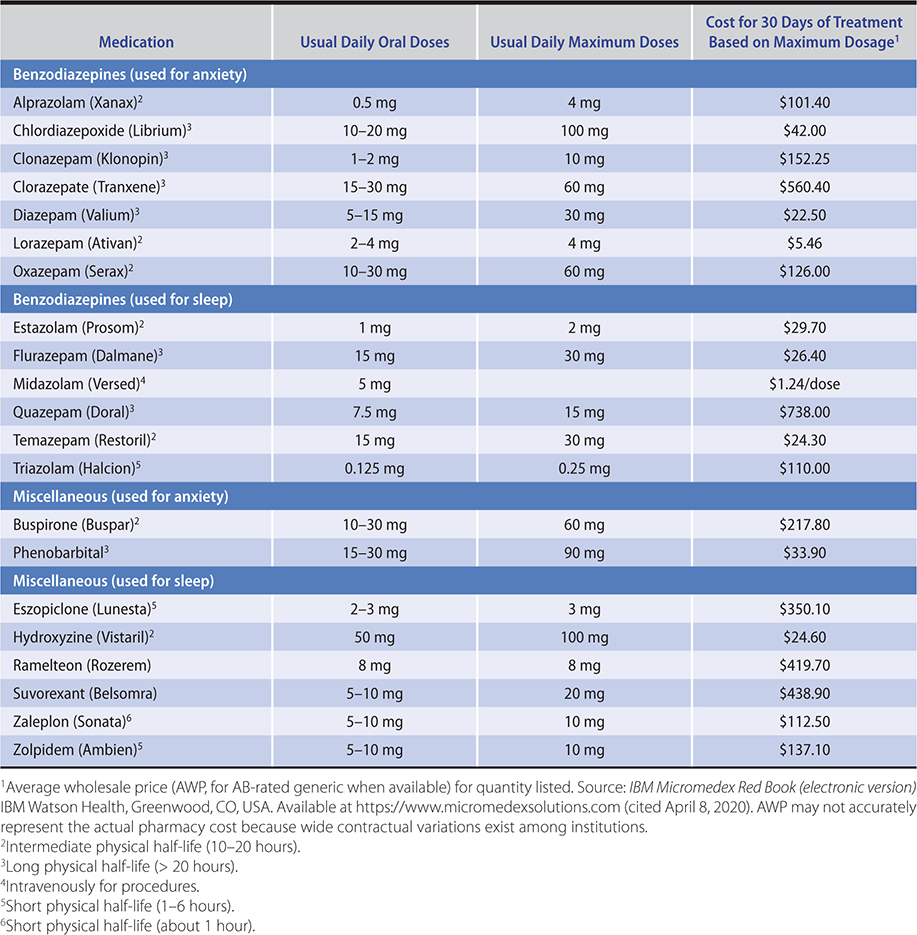
Antidepressants are the first-line medications for sustained treatment of GAD, having the advantage of not causing physiologic dependency problems. Antidepressants can themselves be anxiogenic when first started—thus, at the initiation of treatment, patient education and at times concomitant short-term treatment with a benzodiazepine are indicated. SSRIs, such as escitalopram and paroxetine, are FDA-approved. The SNRIs venlafaxine and duloxetine are FDA-approved for the treatment of GAD in usual antidepressant doses. Initial daily dosing should start low (37.5–75 mg for venlafaxine and 30 mg for duloxetine) and be titrated upward as needed. While most antidepressants including tricyclic antidepressants (TCAs) and monoamine oxidase (MAO) inhibitors are often effective in the treatment of anxiety disorders, their side effects and drug interactions make them second- or third-line agents. Buspirone, sometimes used as an augmenting agent in the treatment of depression and compulsive behaviors, is also effective for generalized anxiety. Buspirone is usually given in a total dose of 30–60 mg/day in divided doses. Higher doses are sometimes associated with side effects of gastrointestinal symptoms and dizziness. Bupropion may be the most anxiogenic antidepressant and does not have evidence in treatment of anxiety disorders. There is a 2- to 4-week delay before antidepressants and buspirone take effect, and patients require education regarding this lag. Sleep is sometimes negatively affected. Gabapentin (titrated to doses of 900–1800 mg orally daily, with larger doses at night) appears effective and lacks the habit-forming potential of the benzodiazepines. Unfortunately, like buspirone, many patients find gabapentin less effective than benzodiazepines in the management of acute anxiety. Beta-blockers, such as propranolol, may help reduce peripheral somatic symptoms. Alcohol is the most frequently self-administered drug and should be strongly discouraged.
2. Panic disorder—Antidepressants are the first-line pharmacotherapy for panic disorder. Several SSRIs, including fluoxetine, paroxetine, and sertraline, are approved for the treatment of panic disorder. The SNRI venlafaxine is FDA approved for treatment of panic disorder. As with GAD, panic disorder is often a chronic condition; the long-term use of benzodiazepines can result in tolerance or even benzodiazepine dependence. While panic disorder often responds to high-potency benzodiazepines such as clonazepam and alprazolam, the best use of these agents is generally early in the course of treatment concurrently with an antidepressant. Once the antidepressant has begun working after 4 or more weeks, the benzodiazepine may be tapered.
Whether the indications for benzodiazepines are anxiety or insomnia, the medications should be used judiciously. The longer-acting benzodiazepines are used for the treatment of alcohol withdrawal and anxiety symptoms; the intermediate medications are useful as sedatives for insomnia (eg, lorazepam), while short-acting agents (eg, midazolam) are used for medical procedures such as endoscopy. Benzodiazepines may be given orally, and several are available in intramuscular or parenteral formulations. In psychiatric disorders, the benzodiazepines are usually given orally; in controlled medical environments (eg, the intensive care unit [ICU]), where the rapid onset of respiratory depression can be assessed, they often are given intravenously. Lorazepam does not produce active metabolites and has a half-life of 10–20 hours; these characteristics are useful in treating elderly patients or those with liver dysfunction. Ultra–short-acting agents, such as triazolam, have half-lives of 1–3 hours and may lead to rebound withdrawal anxiety. Longer-acting benzodiazepines, such as flurazepam, diazepam, and clonazepam, produce active metabolites, have half-lives of 20–120 hours, and should be avoided in the elderly; however, some clinicians prefer clonazepam because of its long half-life and thus ease of dosing to once or twice a day. Since people vary widely in their response and since the medications are long lasting, the dosage must be individualized. Once this is established, an adequate and scheduled dose early in the course of symptom development will obviate the need for “pill popping,” which can contribute to dependency problems.
The side effects of all the benzodiazepine antianxiety agents are patient and dose dependent. As the dosage exceeds the levels necessary for sedation, the side effects include disinhibition, ataxia, dysarthria, nystagmus, and delirium. (The patient should be told not to operate machinery and drive with caution until he or she is well stabilized without side effects.)
Paradoxical agitation, anxiety, psychosis, confusion, mood lability, and anterograde amnesia have been reported, particularly with the shorter-acting benzodiazepines. These agents produce cumulative clinical effects with repeated dosage (especially if the patient has not had time to metabolize the previous dose), additive effects when given with other classes of sedatives or alcohol, and residual effects after termination of treatment (particularly in the case of medications that undergo slow biotransformation).
Overdosage results in respiratory depression, hypotension, shock syndrome, coma, and death. Flumazenil, a benzodiazepine antagonist, is effective in overdosage. Overdosage (see Chapter 38) and withdrawal states are medical emergencies. Serious side effects of chronic excessive dosage are development of tolerance, resulting in increasing dose requirements, and physiologic dependence, resulting in withdrawal symptoms similar in appearance to alcohol and barbiturate withdrawal (withdrawal effects must be distinguished from reemergent anxiety). Abrupt withdrawal of sedative medications may cause serious and even fatal convulsive seizures. Psychosis, delirium, and autonomic dysfunction have also been described. Both duration of action and duration of exposure are major factors related to likelihood of withdrawal.
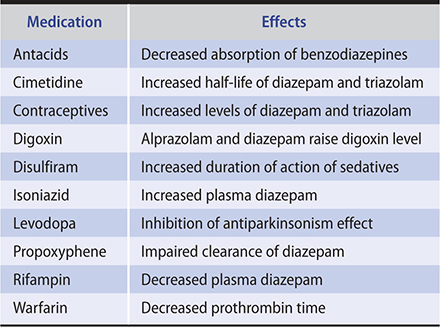
Common withdrawal symptoms after low to moderate daily use of benzodiazepines are classified as somatic (disturbed sleep, tremor, nausea, muscle aches), psychological (anxiety, poor concentration, irritability, mild depression), or perceptual (poor coordination, mild paranoia, mild confusion). The presentation of symptoms will vary depending on the half-life of the medication. Benzodiazepine interactions with other medications are listed in Table 25–2.
Table 25–2. Benzodiazepine interactions with other medications (listed in alphabetical order).
Antidepressants have been used in conjunction with beta-blockers in resistant cases. Propranolol (40–160 mg/day orally) can mute the peripheral symptoms of anxiety without significantly affecting motor and cognitive performance. They block symptoms mediated by sympathetic stimulation (eg, palpitations, tremulousness) but not nonadrenergic symptoms (eg, diarrhea, muscle tension). Contrary to common belief, they usually do not cause depression as a side effect and can be used cautiously in patients with depression.
3. Phobic disorders—Social phobias and agoraphobia may be treated with SSRIs, such as paroxetine, sertraline, and fluvoxamine. In addition, phobic disorders often respond to SNRIs such as venlafaxine. Gabapentin is an alternative to antidepressants in the treatment of social phobia in a dosage of 300–3600 mg/day, depending on response versus sedation. Specific phobias such as performance or test anxiety may respond to moderate doses of beta-blockers, such as propranolol, 20–40 mg 1 hour prior to exposure. Specific phobias tend to respond to behavioral therapies such as systematic desensitization, which is when the patient is gradually exposed to the feared object or situation in a controlled setting.
B. Behavioral
Behavioral approaches are widely used in various anxiety disorders, often in conjunction with medication. Any of the behavioral techniques can be used beneficially in altering the contingencies (precipitating factors or rewards) supporting any anxiety-provoking behavior. Relaxation techniques can sometimes be helpful in reducing anxiety. Desensitization, by exposing the patient to graded doses of a phobic object or situation, is an effective technique and one that the patient can practice outside the therapy session. Emotive imagery, wherein the patient imagines the anxiety-provoking situation while at the same time learning to relax, helps decrease the anxiety when the patient faces the real-life situation. Physiologic symptoms in panic attacks respond well to relaxation training. Both GAD and panic disorder appear to respond as well to cognitive behavioral therapy as they do to medications. Exercise, both aerobic and resistance training, have demonstrated effects in reducing anxiety symptoms across many anxiety disorders as well.
C. Psychological
Cognitive behavioral therapy is the first-line psychotherapy in treatment of anxiety disorders. Cognitive behavioral therapy for anxiety disorders includes a cognitive component of examining the thoughts associated with the fear, and a behavioral technique of exposing the individual to the feared object or situation. The combination of medication and cognitive behavioral therapy is more effective than either alone. Mindfulness meditation can also be effective in decreasing symptoms of anxiety. Group therapy is the treatment of choice when the anxiety is clearly a function of the patient’s difficulties in dealing with social settings. Acceptance and commitment therapy have been used with some success in anxiety disorders. It encourages individuals to keep focused on life goals while they “accept” the presence of anxiety in their lives.
D. Social
Peer support groups for panic disorder and agoraphobia have been particularly helpful. Social modification may require measures such as family counseling to aid acceptance of the patient’s symptoms and avoid counterproductive behavior in behavioral training. Any help in maintaining the social structure is anxiety-alleviating, and work, school, and social activities should be maintained. School and vocational counseling may be provided by professionals, who often need help from the clinician in defining the patient’s limitations.
 Prognosis
Prognosis
Anxiety disorders are usually long-standing and may be difficult to treat. All can be relieved to varying degrees with medications and behavioral techniques. The prognosis is better if the commonly observed anxiety-panic-phobia-depression cycle can be broken with a combination of the therapeutic interventions discussed above.
Carpenter JK et al. Cognitive behavioral therapy for anxiety and related disorders: a meta-analysis of randomized placebo-controlled trials. Depress Anxiety. 2018 Jun;35(6):502–14. [PMID: 29451967]
LeBouthillier DM et al. The efficacy of aerobic exercise and resistance training as transdiagnostic interventions for anxiety-related disorders and constructs: a randomized controlled trial. J Anxiety Disord. 2017 Dec;52:43–52. [PMID: 29049901]
Stein MB et al. Treating anxiety in 2017: optimizing care to improve outcomes. JAMA. 2017 Jul 18;318(3):235–6. [PMID: 28679009]
OBSESSIVE-COMPULSIVE DISORDER & RELATED DISORDERS
ESSENTIALS OF DIAGNOSIS

 Preoccupations or rituals (repetitive psychologically triggered behaviors) that are distressing to the individual.
Preoccupations or rituals (repetitive psychologically triggered behaviors) that are distressing to the individual.
 Symptoms are excessive or persistent beyond potentially developmentally normal periods.
Symptoms are excessive or persistent beyond potentially developmentally normal periods.
 General Considerations
General Considerations
Obsessive-compulsive disorder (OCD), classified as an anxiety disorder in the DSM-IV, now is part of a separate category of Obsessive-Compulsive Disorder and Related Disorders in DSM-5. In OCD, the irrational idea or impulse repeatedly and unwantedly intrudes into awareness. Obsessions (recurring distressing thoughts, such as fears of exposure to germs) and compulsions (repetitive actions such as washing one’s hands many times or cognitions such as counting rituals) are usually recognized by the individual as unwanted or unwarranted and are resisted, but anxiety often is alleviated only by ritualistic performance of the compulsion or by deliberate contemplation of the intruding idea or emotion. Some patients with OCD only experience obsessions, while some experience both obsessions and compulsions. Many patients do not volunteer the symptoms and must be asked about them. There is an overlapping of OCD with some features in other disorders (“OCD spectrum”), including tics, trichotillomania (hair pulling), excoriation disorder (skin picking), hoarding, and body dysmorphic disorder. The incidence of OCD in the general population is 2–3% and there is a high comorbidity with major depression: major depression will develop in two-thirds of OCD patients during their lifetime. Male-to-female ratios are similar, with the highest rates occurring in the young, divorced, separated, and unemployed (all high-stress categories). Neurologic abnormalities of fine motor coordination and involuntary movements are common. Under extreme stress, these patients sometimes exhibit paranoid and delusional behaviors, often associated with depression, and can mimic schizophrenia.
 Treatment
Treatment
A. Pharmacologic
OCD responds to serotonergic antidepressants including SSRIs and clomipramine in about 60% of cases and usually requires a longer time to response than depression (up to 12 weeks). Clomipramine has proved effective in doses equivalent to those used for depression. Fluoxetine has been widely used in this disorder but in doses higher than those used in depression (up to 60–80 mg orally daily). The other SSRI medications, such as sertraline, paroxetine, and fluvoxamine, are used with comparable efficacy each with its own side-effect profile. There is some evidence that antipsychotics and topiramate may be helpful as adjuncts to the SSRIs in treatment-resistant cases. Alternatively, low-dose clomipramine may be an effective adjunct to an SSRI in some patients, though caution should be used when prescribing multiple serotonergic agents given the risk of serotonin syndrome. Plasma levels of clomipramine and its metabolite should be checked 2–3 weeks after a dosing of 50 mg/day has been achieved, with levels being kept under 500 ng/mL to avoid toxicity. Preliminary studies have suggested a role for ketamine and esketamine in the treatment of OCD. Small randomized trials have suggested up to 50% of patients get some relief of their OCD symptoms with in 1 week of a ketamine infusion. Unfortunately, the effects of ketamine on OCD are short-lived and further studies are required to confirm efficacy and optimal dosing.
B. Behavioral
OCD may respond to a variety of behavioral techniques. One common strategy is exposure and response prevention. As in the treatment of simple phobias, exposure and response prevention involves gradually exposing the OCD spectrum patient to situations that the patient fears, such as perceived germs or situations that a hoarder must part with things they are hoarding. By gradually exposing patients to increasingly stressful situations and helping them manage their anxiety without performing the unwanted behavior, OCD spectrum patients are often able to develop some mastery over the behaviors.
C. Psychological
In addition to behavioral techniques, OCD may respond to psychological therapies including cognitive behavioral therapy in which the patient learns to identify maladaptive cognitions associated with obsessive thoughts and challenge those cognitions. For example, a patient with OCD may fear that if he does not wash his hands 50 times after shaking hands, he or someone close to him might develop a serious disease. These cognitions can be identified and gradually replaced with more rational thoughts. A technique used to help quell obsessive thoughts is “thought stopping.” In this technique, the patient is taught to identify an obsessive thought and then to derail it. For example, the patient may be taught to say “STOP” any time an obsessive thought is present. In time, thought stopping can mitigate some of the obsessive thoughts.
D. Social
OCD can have devastating effects on the ability of a patient to lead a normal life. Educating both the patient and family about the course of illness and treatment options is extremely useful in setting appropriate expectations. Severe OCD is commonly associated with vocational disability, and the clinician may sometimes need to facilitate a leave of absence from work or encourage vocational rehabilitation to get the patient back to work.
E. Procedures
Transcranial magnetic stimulation also is effective and FDA-approved for OCD. Psychosurgery has a limited place in selected cases of severe unremitting OCD. Experimental work suggests a role for deep brain stimulation in OCD, and it is FDA approved on a humanitarian device exemption basis for refractory OCD patients.
 Prognosis
Prognosis
OCD is usually a chronic disorder with a waxing and waning course. As many as 40% of patients in whom OCD problems develop in childhood will experience remission as adults. However, it is less common for OCD to remit without treatment when it develops during adulthood.
Carmi L et al. Efficacy and safety of deep transcranial magnetic stimulation for obsessive-compulsive disorder: a prospective multicenter randomized double-blind placebo-controlled trial. Am J Psychiatry. 2019 Nov 1;176(11):931–8. [PMID: 31109199]
Hirschtritt ME et al. Obsessive-compulsive disorder: advances in diagnosis and treatment. JAMA. 2017 Apr 4;317(13):1358–67. [PMID: 28384832]
Rodriguez CI et al. Randomized controlled crossover trial of ketamine in obsessive-compulsive disorder: proof-of-concept. Neuropsychopharmacology. 2013 Nov;38(12):2475–83. [PMID: 23783065]
FEEDING & EATING DISORDERS
See Chapter 29.
SOMATIC SYMPTOM DISORDERS (Abnormal Illness Behaviors)
ESSENTIALS OF DIAGNOSIS

 Prominent physical symptoms may involve one or more organ systems and are associated with distress, impairment, or both.
Prominent physical symptoms may involve one or more organ systems and are associated with distress, impairment, or both.
 Sometimes able to correlate symptom development with psychosocial stresses.
Sometimes able to correlate symptom development with psychosocial stresses.
 Combination of biogenetic and developmental patterns.
Combination of biogenetic and developmental patterns.
 General Considerations
General Considerations
Any organ system can be affected in somatic symptom disorders. In DSM-5, somatic symptom disorders encompass disorders that were listed under somatic disorders in DSM-IV, including conversion disorder, hypochondriasis, somatization disorder, and pain disorder secondary to psychological factors. Vulnerability in one or more organ systems and exposure to family members with somatization problems plays a major role in the development of particular symptoms, and the “functional” versus “organic” dichotomy is a hindrance to good treatment. Clinicians should suspect psychiatric disorders in a number of conditions. For example, 45% of patients describing palpitations had lifetime psychiatric diagnoses including generalized anxiety, depression, panic, and somatic symptom disorders. Similarly, 33–44% of patients who undergo coronary angiography for chest pain but have negative results have been found to have panic disorder.
In any patient presenting with a condition judged to be somatic symptom disorder, depression must be considered in the diagnosis.
 Clinical Findings
Clinical Findings
A. Conversion Disorder (Functional Neurologic Symptom Disorder)
“Conversion” of psychic conflict into physical neurologic symptoms in parts of the body innervated by the sensorimotor system (eg, paralysis, aphonic) is a disorder that commonly occurs concomitantly with panic disorder or depression. The somatic manifestation that takes the place of anxiety is often paralysis, and in some instances the dysfunction may have symbolic meaning (eg, arm paralysis in marked anger so the individual cannot use the arm to strike someone). Nonepileptic seizures can be difficult to differentiate from intoxication states or panic attacks and can occur in patients who also have epileptic seizures. Lack of postictal confusion, closed eyes during the seizure, ictal crying, and a fluctuating course can suggest nonepileptic seizures; some symptoms such as asynchronous movements or pelvic thrusting can occur in both nonepileptic seizures and frontal lobe seizures (see also Chapter 24).
Electroencephalography, particularly in a video-electroencephalography assessment unit, during the attack is the most helpful diagnostic aid in excluding epileptic seizures. A serum prolactin levels rise more than twice baseline abruptly in the postictal state is more likely to be associated with an epileptic seizure. La belle indifférence (an unconcerned affect) is not a significant identifying characteristic, as commonly believed, since individuals even with genuine medical illness may exhibit a high level of denial. It is important to identify physical disorders with unusual presentations (eg, multiple sclerosis, systemic lupus erythematosus).
B. Somatic Symptom Disorder
Somatic symptom disorder is characterized by one or more somatic symptoms that are associated with significant distress or disability. The somatic symptoms are associated with disproportionate and persistent thoughts about the seriousness of the symptoms, a high level of anxiety about health, or excessive time and energy devoted to these symptoms. The patient’s focus on somatic symptoms is usually chronic. Panic, anxiety, and depression are often present, and major depression is an important consideration in the differential diagnosis. There is a significant relationship (20%) to a lifetime history of panic-agoraphobia-depression. It usually occurs before age 30 and is ten times more common in women. Preoccupation with medical and surgical therapy becomes a lifestyle that may exclude other activities. Patients most often first present to primary care physicians and experience reassurance regarding their physical condition as only briefly helpful or dismissive. Patients’ complaints of symptoms should always be first carefully medically evaluated.
C. Factitious Disorders
These disorders, in which symptom production is intentional, are not somatic symptom conditions in that symptoms are produced consciously, in contrast to the unconscious process of the other somatic symptom disorders. They are characterized by self-induced or described symptoms or false physical and laboratory findings for the purpose of deceiving clinicians or other health care personnel. The deceptions may involve self-mutilation, fever, hemorrhage, hypoglycemia, seizures, and an almost endless variety of manifestations—often presented in an exaggerated and dramatic fashion (Munchausen syndrome). Factitious disorder imposed on another, previously termed Munchausen by proxy, is diagnosed when someone (often a parent) creates an illness in another person (often a child) for perceived psychological benefit of the first person, such as sympathy or a relationship with clinicians. The duplicity may be either simple or extremely complex and difficult to recognize. The patients are frequently connected in some way with the health professions and there is no apparent external motivation other than achieving the patient role. A poor clinician-patient relationship and “doctor shopping” tend to exacerbate the problem.
 Complications
Complications
Sedative and analgesic dependency is the most common iatrogenic complication. Patients may pursue medical or surgical treatments that induce iatrogenic problems. Thus, identifying patients with a potential somatic symptom disorder and attempting to limit tests, procedures, and medications that may lead to harm are quite important.
 Treatment
Treatment
A. Medical
Medical support with careful attention to building a therapeutic clinician-patient relationship is the mainstay of treatment. It must be accepted that the patient’s distress is real. Every problem not found to have an organic basis is not necessarily a mental disease. Diligent attempts should be made to relate symptoms to adverse developments in the patient’s life. It may be useful to have the patient keep a meticulous diary, paying particular attention to various pertinent factors evident in the history. Regular, frequent, short appointments that are not symptom-contingent may be helpful. Medications (frequently abused) should not be prescribed to replace appointments. One person should be the primary clinician, and consultants should be used mainly for evaluation. An empathic, realistic, optimistic approach must be maintained in the face of the expected ups and downs. Ongoing reevaluation is necessary, since somatization can coexist with a concurrent physical illness.
B. Psychological
The primary clinician can use psychological approaches when it is clear that the patient is ready to make some changes in lifestyle in order to achieve symptomatic relief. This is often best approached with orientation toward pragmatic current changes rather than an exploration of early experiences that the patient frequently fails to relate to current distress. Cognitive behavioral therapy has been shown to be an effective treatment for somatoform disorders by reducing physical symptoms, psychological distress, and disability. Group therapy with other individuals who have similar problems is sometimes of value to improve coping, allow ventilation, and focus on interpersonal adjustment. Hypnosis used early can be helpful in resolving conversion disorders. If the primary clinician has been working with the patient on psychological problems related to the physical illness, the groundwork is often laid for successful psychiatric referral.
For patients who have been identified as having a factitious disorder, early psychiatric consultation is indicated. There are two main treatment strategies for these patients. One consists of a conjoint confrontation of the patient by both the primary clinician and the psychiatrist. The patient’s disorder is portrayed as a cry for help, and psychiatric treatment is recommended. The second approach avoids direct confrontation and attempts to provide a face-saving way to relinquish the symptom without overt disclosure of the disorder’s origin. Techniques such as biofeedback and self-hypnosis may foster recovery using this strategy.
C. Behavioral
Behavioral therapy is probably best exemplified by biofeedback techniques. In biofeedback, the particular abnormality (eg, increased peristalsis) must be recognized and monitored by the patient and therapist (eg, by an electronic stethoscope to amplify the sounds). This is immediate feedback, and after learning to recognize it, the patient can then learn to identify any change thus produced (eg, a decrease in bowel sounds) and so become a conscious originator of the feedback instead of a passive recipient. Relief of the symptom operantly conditions the patient to utilize the maneuver that relieves symptoms (eg, relaxation causing a decrease in bowel sounds). With emphasis on this type of learning, the patient is able to identify symptoms early and initiate the countermaneuvers, thus decreasing the symptomatic problem. Migraine and tension headaches have been particularly responsive to biofeedback methods.
D. Social
Social endeavors include family, work, and other interpersonal activity. Family members should come for some appointments with the patient so they can learn how best to live with the patient. This is particularly important in treatment of somatic and pain disorders. Peer support groups provide a climate for encouraging the patient to accept and live with the problem. Ongoing communication with the employer may be necessary to encourage long-term continued interest in the employee. Employers can become just as discouraged as clinicians in dealing with employees who have chronic problems.
 Prognosis
Prognosis
The prognosis is better if the primary clinician intervenes early before the situation has deteriorated. After the problem has crystallized into chronicity, it is more difficult to effect change.
den Boeft M et al. How should we manage adults with persistent unexplained physical symptoms? BMJ. 2017 Feb 8;356:j268. [PMID: 28179237]
Henningsen P. Management of somatic symptom disorder. Dialogues Clin Neurosci. 2018 Mar;20(1):23–31. [PMID: 29946208]
Liu J et al. The efficacy of cognitive behavioural therapy in somatoform disorders and medically unexplained physical symptoms: a meta-analysis of randomized controlled trials. J Affect Disord. 2019 Feb 15;245:98–112. [PMID: 30368076]
CHRONIC PAIN DISORDERS
ESSENTIALS OF DIAGNOSIS

 Chronic complaints of pain.
Chronic complaints of pain.
 Symptoms frequently exceed signs.
Symptoms frequently exceed signs.
 Minimal relief with standard treatment.
Minimal relief with standard treatment.
 History of having seen many clinicians.
History of having seen many clinicians.
 Frequent use of several nonspecific medications.
Frequent use of several nonspecific medications.
 General Considerations
General Considerations
A problem in the management of pain is the lack of distinction between acute and chronic pain syndromes. Most clinicians are adept at dealing with acute pain problems but face greater challenges in treating a patient with a chronic pain disorder. Patients with chronic pain can frequently take many medications, stay in bed a great deal, have seen many clinicians, have lost skills, and experience little joy in either work or play. Relationships suffer (including those with clinicians), and life becomes a constant search for relief. The search results in complex clinician-patient relationships that usually include many medication trials, particularly sedatives, with adverse consequences (eg, irritability, depressed mood) related to long-term use. Treatment failures can provoke angry responses and depression from both the patient and the clinician, and the pain syndrome is exacerbated. When frustration becomes too great, a new clinician is found, and the cycle is repeated. The longer the existence of the pain disorder, the more important become the psychological factors of anxiety and depression. As with all other conditions, it is counterproductive to speculate about whether the pain is “real.” It is real to the patient, and acceptance of the problem must precede a mutual endeavor to alleviate the disturbance.
 Clinical Findings
Clinical Findings
Components of the chronic pain syndrome consist of anatomic changes, chronic anxiety and depression, anger, and changed lifestyle. Usually, the anatomic problem is irreversible, since it has already been subjected to many interventions with increasingly unsatisfactory results. An algorithm for assessing chronic pain and differentiating it from other psychiatric conditions is illustrated in Figure 25–1.
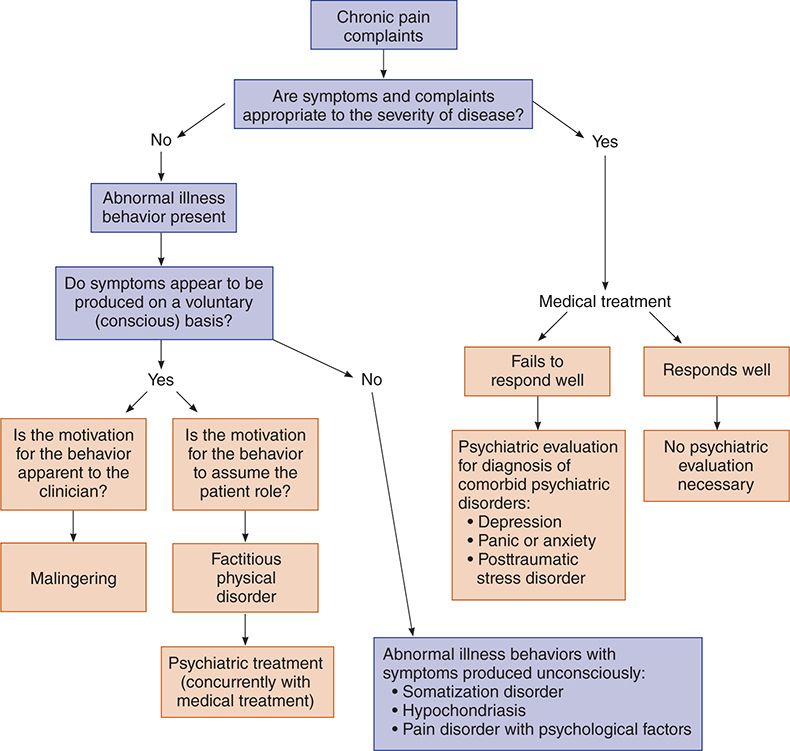
Figure 25–1. Algorithm for assessing psychiatric component of chronic pain. (Adapted and reproduced, with permission, from Eisendrath SJ. Psychiatric aspects of chronic pain. Neurology. 1995 Dec;45(12 Suppl 9):S26–34.)
Chronic anxiety and depression produce heightened irritability and overreaction to stimuli. A marked decrease in pain threshold is apparent. This pattern develops into a preoccupation with the body and a constant need for reassurance. Patients may have started avoiding usual behaviors when they first developed pain, and then chronic avoidance of usual physical functioning can lead to the development of chronic pain. The pressure on the clinician becomes wearing and often leads to covert rejection of the patient, such as not being available or making referrals to other clinicians.
This is perceived by the patient, who then intensifies the effort to find help, and the typical cycle is repeated. Anxiety and depression are seldom discussed, almost as if there is a tacit agreement not to deal with these issues.
Changes in lifestyle involve some of the pain behaviors. These usually take the form of a family script in which the patient accepts the role of being sick, and this role then becomes the focus of most family interactions and may become important in maintaining the family, so that neither the patient nor the family wants the patient’s role to change. Cultural factors frequently play a role in the behavior of the patient and how the significant people around the patient cope with the problem. Some cultures encourage demonstrative behavior, while others value the stoic role.
Another secondary gain that can maintain the patient in the sick role is financial compensation or other benefits. Frequently, such systems are structured so that they reinforce the maintenance of sickness and discourage any attempts to give up the role. Clinicians unwittingly reinforce this role because of the very nature of the practice of medicine, which is to respond to complaints of illness. Helpful suggestions from the clinician are often met with responses like, “Yes, but….” Medications then become the principal approach, and drug dependency problems may develop.
 Treatment
Treatment
A. Behavioral
The cornerstone of a unified approach to chronic pain syndromes is a comprehensive behavioral program. This is necessary to identify and eliminate pain reinforcers, to decrease medication use, and to use effectively those positive reinforcers that shift the focus from the pain. It is critical that the patient be made a partner in the effort to manage and function better in the setting of ongoing pain symptoms. The clinician must shift from the idea of biomedical cure to ongoing care of the patient. The patient should agree to discuss the pain only with the clinician and not with family members; this tends to stabilize the patient’s personal life, since the family is usually tired of the subject. At the beginning of treatment, the patient should be assigned self-help tasks graded up to maximal activity as a means of positive reinforcement. The tasks should not exceed capability. The patient can also be asked to keep a self-rating chart to log accomplishments, so that progress can be measured and remembered. Instruct the patient to record degrees of pain on a self-rating scale in relation to various situations and mental attitudes so that similar circumstances can be avoided or modified.
Avoid positive reinforcers for pain such as marked sympathy and attention to pain. Emphasize a positive response to productive activities, which remove the focus of attention from the pain. Activity is also desensitizing, since the patient learns to tolerate increasing activity levels.
Biofeedback techniques (see Somatic Symptom Disorders, above) and hypnosis have been successful in ameliorating some pain syndromes. Hypnosis tends to be most effective in patients with a high level of denial, who are more responsive to suggestion. Hypnosis can be used to lessen anxiety, alter perception of the length of time that pain is experienced, and encourage relaxation. Mindfulness-based stress reduction programs have been useful in helping individuals develop an enhanced capacity to live a higher quality life with persistent pain.
B. Medical
A single clinician in charge of the comprehensive treatment approach is the highest priority. Consultations as indicated and technical procedures done by others are appropriate, but the care of the patient should remain in the hands of the primary clinician. Referrals should not be allowed to raise the patient’s hopes unrealistically or to become a way for the clinician to reject the case. The attitude of the clinician should be one of honesty, interest, and hopefulness—not for a cure but for control of pain and improved function. If the patient manifests opioid addiction, detoxification may be an early treatment goal.
Medical management of chronic pain is addressed in Chapter 5. The harms of opioids generally outweigh the benefits in chronic pain management. A fixed schedule lessens the conditioning effects of these medications. SNRIs (eg, venlafaxine, milnacipran, and duloxetine) and TCAs (eg, nortriptyline) in doses up to those used in depression may be helpful, particularly in neuropathic pain syndromes. Both duloxetine and milnacipran are approved for the treatment of fibromyalgia; duloxetine is also indicated in chronic pain conditions. In general, the SNRIs tend to be safer in overdose than the TCAs (although venlafaxine may be more arrhythmogenic in overdose than other SRNIs but less than TCAs); suicidality is often an important consideration in treating patients with chronic pain syndromes. Gabapentin and pregabalin, anticonvulsants with possible applications in the treatment of anxiety disorders, have been shown to be useful in neuropathic pain and fibromyalgia.
In addition to medications, a variety of nonpharmacologic strategies may be offered, including physical therapy and acupuncture.
C. Social
Involvement of family members and other significant persons in the patient’s life should be an early priority. The best efforts of both patient and therapists can be unwittingly sabotaged by other persons who may feel that they are “helping” the patient. They frequently tend to reinforce the negative aspects of the chronic pain disorder. The patient becomes more dependent and less active, and the pain syndrome becomes an immutable way of life. The more destructive pain behaviors described by many experts in chronic pain disorders are the results of well-meaning but misguided efforts of family members. Ongoing therapy with the family can be helpful in the early identification and elimination of these behavior patterns.
D. Psychological
In addition to group therapy with family members and others, groups of patients can be helpful if properly led. The major goal, whether of individual or group therapy, is to gain patient involvement. A group can be a powerful instrument for achieving this goal, with the development of group loyalties and cooperation. People will frequently make efforts with group encouragement that they would never make alone. Individual therapy should be directed toward strengthening existing coping mechanisms and improving self-esteem. For example, teaching patients to challenge expectations induced by chronic pain may lead to improved functioning. As an illustration, many chronic pain patients, making assumptions more derived from acute injuries, incorrectly believe they will damage themselves by attempting to function. The rapport between patient and clinician, as in all psychotherapeutic efforts, is the major factor in therapeutic success.
Busse JW et al. Guideline for opioid therapy and chronic noncancer pain. CMAJ. 2017 May 8;189(18):E659–66. [PMID: 28483845]
Markozannes G et al. An umbrella review of the literature on the effectiveness of psychological interventions for pain reduction. BMC Psychol. 2017 Aug 31;5(1):31. [PMID: 28859685]
Urits I et al. Off-label antidepressant use for treatment and management of chronic pain: evolving understanding and comprehensive review. Curr Pain Headache Rep. 2019 Jul 29;23(9):66. [PMID: 31359175]
PSYCHOSEXUAL DISORDERS
The stages of sexual activity include excitement (arousal), orgasm, and resolution. The precipitating excitement or arousal is psychologically determined. Arousal response leading to orgasm is a physiologic and psychological phenomenon of vasocongestion, a parasympathetic reaction causing erection in men and labial-clitoral congestion in women. The orgasmic response includes emission in men and clonic contractions of the analogous striated perineal muscles of both men and women. Resolution is a gradual return to normal physiologic status.
While the arousal stimuli—vasocongestive and orgasmic responses—constitute a single response in a well-adjusted person, they can be considered as separate stages that can produce different syndromes responding to different treatment procedures.
 Clinical Findings
Clinical Findings
There are three major groups of sexual disorders.
A. Paraphilias
In these conditions, formerly called “deviations” or “variations,” the excitement stage of sexual activity is associated with sexual objects or orientations different from those usually associated with adult sexual stimulation. The stimulus may be a woman’s shoe, a child, animals, instruments of torture, or incidents of aggression. The pattern of sexual stimulation is usually one that has early psychological roots. When paraphilias are associated with distress, impairment, or risk of harm, they become paraphilic disorders. Some paraphilias or paraphilic disorders include exhibitionism, transvestism, voyeurism, pedophilia, incest, sexual sadism, and sexual masochism.
B. Gender Dysphoria
Gender dysphoria is distress associated with the incongruence between one’s experienced or expressed gender and one’s assigned gender. As a disorder, it is defined by significant distress or impairment; those experiencing this incongruence but without the distress would not meet criteria for having gender dysphoria. Screening should be done for conditions related to the oppression and stigmatization that transgender people face, including a high risk of suicide.
C. Sexual Dysfunctions
This category includes a large group of vasocongestive and orgasmic disorders. Often, they involve problems of sexual adaptation, education, and technique that are often initially discussed with, diagnosed by, and treated by the primary care provider.
There are two conditions common in men: erectile dysfunction and ejaculation disturbances.
Erectile dysfunction is inability to achieve or maintain an erection firm enough for satisfactory intercourse; patients sometimes use the term incorrectly to mean premature ejaculation. Decreased nocturnal penile tumescence occurs in some depressed patients. Psychological erectile dysfunction is caused by interpersonal or intrapsychic factors (eg, partner disharmony, depression). Organic factors are discussed in Chapter 23.
Ejaculation disturbances include premature ejaculation, inability to ejaculate, and retrograde ejaculation. (Ejaculation is possible in patients with erectile dysfunction.) Ejaculation is usually connected with orgasm, and ejaculatory control is an acquired behavior that is minimal in adolescence and increases with experience. Pathogenic factors are those that interfere with learning control, most frequently sexual ignorance. Intrapsychic factors (anxiety, guilt, depression) and interpersonal maladaptation (partner problems, unresponsiveness of mate, power struggles) are also common. Organic causes include interference with sympathetic nerve distribution (often due to surgery or radiation) and the effects of pharmacologic agents (eg, SSRIs or sympatholytics).
In women, the most common forms of sexual dysfunction are orgasmic disorder and hypoactive sexual desire disorder.
Orgasmic disorder is a complex condition in which there is a general lack of sexual responsiveness. The woman has difficulty in experiencing erotic sensation and does not have the vasocongestive response. Sexual activity varies from active avoidance of sex to an occasional orgasm. Orgasmic dysfunction—in which a woman has a vasocongestive response but varying degrees of difficulty in reaching orgasm—is sometimes differentiated from anorgasmia. Causes for the dysfunctions include poor sexual techniques, early traumatic sexual experiences, interpersonal disharmony (partner struggles, use of sex as a means of control), and intrapsychic problems (anxiety, fear, guilt). Organic causes include any conditions that might cause pain in intercourse, pelvic pathology, mechanical obstruction, and neurologic deficits.
Hypoactive sexual desire disorder consists of diminished or absent libido in either sex and may be a function of organic or psychological difficulties (eg, anxiety, phobic avoidance). Any chronic illness can reduce desire as can aging. Hormonal disorders, including hypogonadism or use of antiandrogen compounds such as cyproterone acetate, and chronic kidney disease contribute to deterioration in sexual desire. Alcohol, sedatives, opioids, marijuana, and some medications may affect sexual drive and performance. Menopause may lead to diminution of sexual desire in some women, and testosterone therapy is sometimes warranted as treatment.
 Treatment
Treatment
A. Paraphilias
1. Psychological—Paraphilias, particularly those of a more superficial nature (eg, voyeurism) and those of recent onset, are responsive to psychotherapy in some cases. The prognosis is much better if the motivation comes from the individual rather than the legal system; unfortunately, judicial intervention is frequently the only stimulus to treatment because the condition persists and is reinforced until conflict with the law occurs. Therapies frequently focus on barriers to normal arousal response; the expectation is that the variant behavior will decrease as normal behavior increases.
2. Behavioral—In some cases, paraphilic disorders improve with modeling, role-playing, and conditioning procedures.
3. Social—Although they do not produce a change in sexual arousal patterns or gender role, self-help groups have facilitated adjustment to an often hostile society. Attention to the family is particularly important in helping people in such groups to accept their situation and alleviate their guilt about the role they think they had in creating the problem.
4. Pharmacologic—Medroxyprogesterone acetate, a suppressor of libidinal drive, can be used to mute disruptive sexual behavior in men. Onset of action is usually within 3 weeks, and the effects are generally reversible. Fluoxetine or other SSRIs at depression doses may reduce some of the compulsive sexual behaviors including the paraphilias. A focus of study in the treatment of severe paraphilia has been agonists of luteinizing hormone–releasing hormone (LHRH). Case reports and open label studies suggest that LHRH-agonists may play a role in preventing relapse in some patients with paraphilia.
B. Gender Dysphoria
1. Psychological—Individuals with gender dysphoria often find benefit from psychotherapy, providing them with a safe place to explore and understand their thoughts and feelings, and to identify their own specific needs and desires and adjust to a changing life.
2. Social—Peer support groups, parent psychoeducation and support, and community empowerment are important social components of treatment.
3. Medical—Some individuals with gender dysphoria choose to pursue surgery or hormone therapy or both. Most recommendations prior to surgery include that the individual spends significant time prior living as their desired gender. Rates of suicide fall significantly after surgery but still remain much higher than the general population.
C. Sexual Dysfunction
1. Psychological—The use of psychotherapy by itself is best suited for those cases in which interpersonal difficulties or intrapsychic problems predominate. Anxiety and guilt about parental injunctions against sex may contribute to sexual dysfunction. Even in these cases, however, a combined behavioral-psychological approach usually produces results most quickly.
2. Behavioral—Syndromes resulting from conditioned responses have been treated by conditioning techniques, with excellent results. Masters and Johnson have used behavioral approaches in all of the sexual dysfunctions, with concomitant supportive psychotherapy and with improvement of the communication patterns of the couple.
3. Social—The proximity of other people (eg, a mother-in-law) in a household is frequently an inhibiting factor in sexual relationships. In such cases, some social engineering may alleviate the problem.
4. Medical—Even if the condition is not reversible, identification of the specific cause helps the patient to accept the condition. Partner disharmony, with its exacerbating effects, may thus be avoided. Of all the sexual dysfunctions, erectile dysfunction is the condition most likely to have an organic basis. Sildenafil, tadalafil, and vardenafil are phosphodiesterase type 5 inhibitors that are effective oral agents for the treatment of penile erectile dysfunction (eg, sildenafil 25–100 mg orally 1 hour prior to intercourse). These agents are effective for SSRI-induced erectile dysfunction in men and in some cases for SSRI-associated sexual dysfunction in women. Use of the medications in conjunction with any nitrates can have significant hypotensive effects leading to death in rare cases. Because of their common effect in delaying ejaculation, the SSRIs have been effective in premature ejaculation.
Flibanserin is a 5-HT1A-agonist/5-HT2-antagonist that is FDA approved for the treatment of female hypoactive sexual desire disorder. Women treated with flibanserin have a marginally higher number of sexual events. The medication interacts with alcohol, causing hypotensive events, so patients need to be educated about this risk. Flibanserin is taken 100 mg orally at bedtime to circumvent the side effects of dizziness, sleepiness, and nausea.
In addition to flibanserin, a second medication was approved by the FDA in 2019 for the treatment of hypoactive sexual desire disorder in premenopausal women. Bremelanotide activates melanocortin receptors, although the mechanism of action in hypoactive sexual desire disorder is unclear. It is self-administered by injection to the thigh or abdomen about 45 minutes before anticipated sexual activity. In the registration trials, about 25% of women had a clinically significant increase in libido compared to 17% of controls. While the response rates appear low, bremelanotide was generally well tolerated and does represent another option for some women with hypoactive sexual desire disorder.
Dhillon S et al. Bremelanotide: first approval. Drugs. 2019 Sep;79(14):1599–606. [PMID: 31429064]
Hadj-Moussa M et al. Evaluation and treatment of gender dysphoria to prepare for gender confirmation surgery. Sex Med Rev. 2018 Oct;6(4):607–17. [PMID: 29891226]
PERSONALITY DISORDERS
ESSENTIALS OF DIAGNOSIS

 Long history dating back to childhood.
Long history dating back to childhood.
 Recurrent maladaptive behavior.
Recurrent maladaptive behavior.
 Difficulties with interpersonal relationships or society.
Difficulties with interpersonal relationships or society.
 Depression with anxiety when maladaptive behavior fails.
Depression with anxiety when maladaptive behavior fails.
 General Considerations
General Considerations
An individual’s personality structure, or character, is an integral part of self-image. It reflects genetics, interpersonal influences, and recurring patterns of behavior adopted to cope with the environment. The classification of subtypes of personality disorders depends on the predominant symptoms and their severity. The most severe disorders—those that bring the patient into greatest conflict with society—tend to be antisocial (psychopathic) or borderline.
 Classification & Clinical Findings
Classification & Clinical Findings
See Table 25–3.
Table 25–3. Personality disorders: Classification and clinical findings (listed in alphabetical order).

 Differential Diagnosis
Differential Diagnosis
Patients with personality disorders tend to experience anxiety and depression when pathologic coping mechanisms fail and may first seek treatment when this occurs. Occasionally, the more severe cases may decompensate into psychosis under stress and mimic other psychotic disorders.
 Treatment
Treatment
A. Social
Social and therapeutic environments such as day hospitals, halfway houses, and self-help communities utilize peer “pressure” to modify the self-destructive behavior. The patient with a personality disorder often has failed to profit from experience, and difficulties with authority can impair the learning experience. The use of peer relationships and the repetition possible in a structured setting of a helpful community enhance the behavioral treatment opportunities and increase learning. When problems are detected early, both the school and the home can serve as foci of intensified social pressure to change the behavior, particularly with the use of behavioral techniques.
B. Behavioral
The behavioral techniques used are principally operant conditioning and aversive conditioning. The former simply emphasizes the recognition of acceptable behavior and its reinforcement with praise or other tangible rewards. Aversive responses usually mean punishment, although this can range from a mild rebuke to some specific punitive responses such as deprivation of privileges. Extinction plays a role in that an attempt is made not to respond to inappropriate behavior, and the lack of response eventually causes the person to abandon that type of behavior. Pouting and tantrums, for example, diminish quickly when such behavior elicits no reaction. These traits are less likely to develop in children at risk for development of antisocial tendencies due to a genetic predisposition when they are given positive reinforcement for positive behaviors. Dialectical behavioral therapy is a program of individual and group therapy specifically designed for patients with chronic suicidality and borderline personality disorder. It blends mindfulness and a cognitive-behavioral model to address self-awareness, interpersonal functioning, affective lability, and reactions to stress. Psychodynamic psychotherapy can also be an effective treatment.
C. Psychological
Psychological interventions can be conducted in group and individual settings. Group therapy is helpful when specific interpersonal behavior needs to be improved. This mode of treatment also has a place with so-called “acting-out” patients, ie, those who frequently act in an impulsive and inappropriate way. The peer pressure in the group tends to impose restraints on rash behavior. The group also quickly identifies the patient’s types of behavior and helps improve the validity of the patient’s self-assessment, so that the antecedents of the unacceptable behavior can be effectively handled, thus decreasing its frequency. Individual therapy should initially be supportive, ie, helping the patient to restabilize and mobilize coping mechanisms. If the individual has the ability to observe his or her own behavior, a longer-term and more introspective therapy may be warranted. The therapist must be able to handle countertransference feelings (which are frequently negative), maintain appropriate boundaries in the relationship, and refrain from premature confrontations and interpretations.
D. Pharmacologic
Hospitalization is indicated in the case of serious suicidal or homicidal danger. In most cases, treatment can be accomplished in the day treatment center or self-help community. Pharmacotherapy can be directed to specific symptom clusters, but there is limited evidence for its efficacy in personality disorders. Antidepressants have improved anxiety, depression, and sensitivity to rejection in some patients with borderline personality disorder. SSRIs also have a role in reducing aggressive behavior in impulsive aggressive patients (eg, fluoxetine 20–60 mg orally daily or sertraline 50–200 mg orally daily). Antipsychotics may be helpful in targeting hostility, agitation, and as adjuncts to antidepressant therapy (eg, olanzapine [2.5–10 mg/day orally], risperidone [0.5–2 mg/day orally], or haloperidol [0.5–2 mg/day orally, split into two doses]). In some cases, these medications are required only for several days and can be discontinued after the patient has regained a previously established level of adjustment; they can also provide ongoing support. Anticonvulsants, including carbamazepine, 400–800 mg orally daily in divided doses, lamotrigine, 50–200 mg/day and valproate 500–2000 mg/day, have been shown to decrease the severity of behavioral dyscontrol in some personality disorder patients. Patients with a schizotypal personality often improve with antipsychotics, while those with avoidant personality may benefit from strategies that reduce anxiety, including the use of SSRIs and benzodiazepines. Intermittent explosive disorder is characterized by episodes of unwarranted anger and sometime violence. Antipsychotics and anticonvulsants have been helpful for some patients with intermittent explosive disorder. In addition, there is some initial evidence that the combination of dextromethorphan and quinidine, which is currently approved for treating pseudobulbar affect, may have a role in the treatment of intermittent explosive disorder. Studies are in progress as of early 2020.
 Prognosis
Prognosis
Antisocial and borderline categories generally have a guarded prognosis. Those patients with a history of parental abuse and a family history of mood disorder tend to have the most challenging treatments.
Cristea IA et al. Efficacy of psychotherapies for borderline personality disorder: a systematic review and meta-analysis. JAMA Psychiatry. 2017 Apr 1;74(4):319–28. [PMID: 28249086]
Faay MDM et al. Efficacy of typical and atypical antipsychotic medication on hostility in patients with psychosis-spectrum disorders: a review and meta-analysis. Neuropsychopharmacology. 2018 Nov;43(12):2340–9. [PMID: 30093698]
Hancock-Johnson E et al. A focused systematic review of pharmacological treatment for borderline personality disorder. CNS Drugs. 2017 May;31(5):345–56. [PMID: 28353141]
Moukaddam N et al. Difficult patients in the emergency department: personality disorders and beyond. Psychiatr Clin North Am. 2017 Sep;40(3):379–95. [PMID: 28800796]
SCHIZOPHRENIA SPECTRUM DISORDERS
ESSENTIALS OF DIAGNOSIS

 Social withdrawal, usually slowly progressive, with decrease in emotional expression or motivation or both.
Social withdrawal, usually slowly progressive, with decrease in emotional expression or motivation or both.
 Deterioration in personal care with disorganized behaviors or decreased reactivity to the environment or both.
Deterioration in personal care with disorganized behaviors or decreased reactivity to the environment or both.
 Disorganized thinking, often inferred from speech that switches topics oddly or is incoherent.
Disorganized thinking, often inferred from speech that switches topics oddly or is incoherent.
 Auditory hallucinations, often of a derogatory nature.
Auditory hallucinations, often of a derogatory nature.
 Delusions, fixed false beliefs despite conflicting evidence, frequently of a persecutory nature.
Delusions, fixed false beliefs despite conflicting evidence, frequently of a persecutory nature.
 General Considerations
General Considerations
Schizophrenia is manifested by a massive disruption of thinking, mood, and overall behavior as well as poor filtering of stimuli. The cause of schizophrenia is believed to be multifactorial, with genetic, environmental, and neurotransmitter pathophysiologic components. At present, there is no laboratory method for confirming the diagnosis of schizophrenia. There may or may not be a history of a major disruption in the individual’s life (failure, loss, physical illness) before gross psychotic deterioration is evident.
Other psychotic disorders on this spectrum are conditions that are similar to schizophrenia in their acute symptoms, but have a less pervasive influence over the long term. The patient usually attains higher levels of functioning. The acute psychotic episodes tend to be less disruptive of the person’s lifestyle, with a fairly quick return to previous levels of functioning.
 Classification
Classification
A. Schizophrenia
Schizophrenia is the most common of the psychotic disorders that are all characterized by a loss of contact with reality. The term psychosis is broad and most often refers to having paranoia, auditory hallucinations, delusions, or all of these symptoms. One percent of the population suffers from schizophrenia. Schizophrenia is a chronic disorder that is characterized by increasing social and vocational disability that begins in late adolescence or early adulthood and tends to continue through life. The average age of onset for men is 18 years and for women is 25 years. Symptoms have been classified into positive and negative categories. Positive symptoms include hallucinations, delusions, and disorganized speech; these symptoms appear to be related to increased dopaminergic (D2) activity in the mesolimbic region, and all patients have at least one or two of these symptoms to meet criteria for diagnosis. There is often a component of paranoia involved. They may also have disorganized behavior, lack of emotional/cognitive responsiveness, or both. Negative symptoms include diminished sociability, restricted affect, and poverty of speech; these symptoms appear to be related to decreased D2 activity in the mesocortical system. Level of functioning is markedly below that before the onset of symptoms, which must last at least 6 months in some form.
B. Delusional Disorder
Delusional disorders are psychoses in which the predominant symptoms are persistent delusions (ie, beliefs that are false yet fixed despite being shown evidence that they are unfounded) with minimal impairment of daily functioning. Intellectual and occupational activities are little affected, whereas social and partner functioning tends to be markedly involved. Hallucinations are not usually present. Common delusional themes include paranoid delusions of persecution, delusions of being related to or loved by a well-known person, and delusions that one’s partner is unfaithful.
C. Schizoaffective Disorder
Schizoaffective disorders are those cases that fail to fit comfortably either in the schizophrenia or in the affective categories. They are usually cases with affective symptoms (either a major depressive episode, manic episode, or hypomanic episode) that precede or develop concurrently with psychotic manifestations, and the psychotic symptoms occur in the absence of any mood symptoms. The psychotic symptoms begin before the mood episode begins and can continue to linger for some time after resolution of the mood episode but do not remain permanently. Because of this, the long-term prognosis is better than for schizophrenia.
D. Schizophreniform Disorders
Schizophreniform disorders are similar in their symptoms to schizophrenic disorders except that the duration of prodromal, acute, and residual symptoms is longer than 1 month but less than 6 months.
E. Brief Psychotic Disorders
Brief psychotic disorders are defined as psychotic symptoms lasting less than 1 month. They are the result of psychological stress. The shorter duration is significant and correlates with a more acute onset and resolution as well as a much better prognosis.
 Clinical Findings
Clinical Findings
A. Symptoms and Signs
The symptoms and signs of schizophrenia vary markedly among individuals as well as in the same person at different times. The patient’s appearance may be bizarre, although the usual finding is mildly to moderately unkempt. Motor activity is generally reduced, although extremes ranging from catatonic stupor to frenzied excitement occur. Social behavior is characterized by marked withdrawal coupled with disturbed interpersonal relationships and a reduced ability to experience pleasure. Dependency and a poor self-image are common. Verbal utterances are variable, the language being concrete yet symbolic, with unassociated rambling statements (at times interspersed with mutism) during an acute episode. Neologisms (made-up words or phrases), echolalia (repetition of words spoken by others), and verbigeration (repetition of senseless words or phrases) are occasionally present. Affect is usually flattened, with occasional inappropriateness. Depression is present in almost all cases but may be less apparent during the acute psychotic episode and more obvious during recovery. Depression is sometimes confused with akinetic side effects of antipsychotic medications. It is also related to boredom, which increases symptoms and decreases the response to treatment. Work is generally unavailable and time unfilled, providing opportunities for counterproductive activities such as drug abuse, withdrawal, and increased psychotic symptoms.
Thought content may vary from a paucity of ideas to a rich complex of delusional fantasy with archaic thinking. One frequently notes after a period of conversation that little if any information has actually been conveyed. Incoming stimuli produce varied responses. In some cases, a simple question may trigger explosive outbursts, whereas at other times there may be no overt response whatsoever (catatonia). When paranoid ideation is present, the patient is often irritable and less cooperative. Delusions (false beliefs) are characteristic of paranoid thinking, and they usually take the form of a preoccupation with the supposedly threatening behavior exhibited by other individuals. This ideation may cause the patient to adopt active countermeasures such as locking doors and windows, taking up weapons, covering the ceiling with aluminum foil to counteract radar waves, and other bizarre efforts. Somatic delusions revolve around issues of bodily decay or infestation. Perceptual distortions usually include auditory hallucinations—visual hallucinations are more commonly associated with organic mental states—and may include illusions (distortions of reality) such as figures changing in size or lights varying in intensity. Cenesthetic hallucinations (eg, a burning sensation in the brain, feeling blood flowing in blood vessels) occasionally occur. Lack of humor, feelings of dread, depersonalization (a feeling of being apart from the self), and fears of annihilation may be present. Any of the above symptoms generate higher anxiety levels, with heightened arousal and occasional panic and suicidal ideation, as the individual fails to cope.
The development of the acute episode in schizophrenia frequently is the end product of a gradual decompensation. Frustration and anxiety appear early, followed by depression and alienation, along with progressive ineffectiveness in day-to-day coping. This often leads to feelings of panic and increasing disorganization, with loss of the ability to test and evaluate the reality of perceptions. The stage of so-called psychotic resolution includes delusions, autistic preoccupations, and psychotic insight, with acceptance of the decompensated state. The process is frequently complicated by the use of caffeine, alcohol, and other recreational drugs. Life expectancy of patients with schizophrenia is as much as 20% shorter than that of cohorts in the general population and is often associated with comorbid conditions such as the metabolic syndrome, which may be induced or exacerbated by the atypical antipsychotic agents.
Polydipsia may produce water intoxication with hyponatremia—characterized by symptoms of confusion, lethargy, psychosis, seizures, and occasionally death—in any psychiatric disorder, but most commonly in schizophrenia. These problems exacerbate the schizophrenic symptoms and can be confused with them. Possible pathogenetic factors in polydipsia include a hypothalamic defect, inappropriate antidiuretic hormone (ADH) secretion, antipsychotic medications (anticholinergic effects, stimulation of hypothalamic thirst center, effect on ADH), smoking (nicotine and syndrome of inappropriate antidiuretic hormone [SIADH]), psychotic thought processes (delusions), and other medications (eg, diuretics, antidepressants, lithium, alcohol) (see Chapter 21).
B. Imaging
Ventricular enlargement and cortical atrophy, as seen on CT scan, have been correlated with chronic course, severe cognitive impairment, and nonresponsiveness to antipsychotic medications. Decreased frontal lobe activity seen on PET scan has been associated with negative symptoms.
 Differential Diagnosis
Differential Diagnosis
The diagnosis of schizophrenia is best made over time because repeated observations increase the reliability of the diagnosis. One should not hesitate to reconsider the diagnosis of schizophrenia in any person who has received that diagnosis in the past, particularly when the clinical course has been atypical. A number of these patients have been found to actually have bipolar disorder, which has responded well to lithium. Manic episodes often mimic schizophrenia. However, schizophrenia is less likely to be associated with the decreased need for sleep, increase in goal-directed activity, and overconfidence, symptoms that are typical of mania. However, thought disorder, auditory hallucinations, and delusions are commonly seen in manic psychosis.
Psychotic depressions, brief reactive psychosis, delusional disorder, and any illness with psychotic ideation tend to be confused with schizophrenia, partly because of the regrettable tendency to use the terms interchangeably.
Medical disorders such as thyroid dysfunction, adrenal and pituitary disorders, reactions to toxic materials (eg, mercury, PCBs), and almost all of the organic mental states in the early stages must be ruled out. Postpartum psychosis is discussed under Mood Disorders. Complex partial seizures, especially when psychosensory phenomena are present, are an important differential consideration. Toxic drug states arising from prescription, over-the-counter, herbal and street drugs may mimic all of the psychotic disorders. The chronic use of amphetamines, cocaine, and other stimulants frequently produces a psychosis that is almost identical to the acute paranoid schizophrenic episode. Drug-induced psychoses can have all the positive symptoms of schizophrenia but less commonly have the negative symptoms. The presence of formication (sensation of insects crawling on or under the skin) and stereotypy suggests the possibility of stimulant abuse. Phencyclidine, a common street drug, may cause a reaction that is difficult to distinguish from other psychotic disorders. Cerebellar signs, excessive salivation, dilated pupils, and increased deep tendon reflexes should alert the clinician to the possibility of a toxic psychosis. Industrial chemical toxicity (both organic and metallic), degenerative disorders, and metabolic deficiencies must be considered in the differential diagnosis.
Catatonia, a psychomotor disturbance that may involve decreased motor activity, decreased interaction, or excessive and odd motor activity, is frequently assumed to exist solely as a component of schizophrenic disorders. However, it can actually be the end product of a number of illnesses, including a number of organic conditions as well as other psychiatric disorders such as bipolar disorder. Neoplasms, viral and bacterial encephalopathies, central nervous system hemorrhage, metabolic derangements such as diabetic ketoacidosis, sedative withdrawal, and liver and kidney malfunction have all been implicated. It is particularly important to realize that drug toxicity (eg, overdoses of antipsychotic medications such as fluphenazine or haloperidol) can cause catatonic syndrome, which may be misdiagnosed as a catatonic schizophrenia and inappropriately treated with more antipsychotic medication. Catatonia is also seen in other major psychiatric disorders, including bipolar disorder and major depression.
 Treatment
Treatment
A. Pharmacologic (see Side Effects, below)
Hospitalization is sometimes necessary, particularly when the patient’s behavior shows gross disorganization. The presence of competent family members or social support lessens the need for hospitalization, and each case should be judged individually. The major considerations are to prevent self-inflicted harm or harm to others and to provide the patient’s basic needs. A full medical evaluation and CT scan or MRI of the brain should be considered in first episodes of psychosis.
Antipsychotic medications are the treatment of choice. The relapse rate can be reduced by 50% with proper maintenance antipsychotic therapy. Long-acting, injectable antipsychotics are used in patients who are not adherent to medication recommendations or who do not respond to oral medication, or patients who choose the ease of not taking a daily pill.
Antipsychotic medications include the “typical or first-generation” antipsychotics (phenothiazines, thioxanthenes, butyrophenones, dihydroindolones, dibenzoxazepines, and benzisoxazoles) and the newer “atypical or second-generation” antipsychotics (clozapine, risperidone, olanzapine, quetiapine, aripiprazole, ziprasidone, paliperidone, asenapine, iloperidone, lurasidone, and cariprazine) (Tables 25–4 and 25–5). Generally, increasing milligram potency of the typical antipsychotics is associated with decreasing anticholinergic and adrenergic side effects and increasing extrapyramidal symptoms. Data suggest similar antipsychotic efficacy for first- and second-generation antipsychotics, but a tendency for the second-generation antipsychotics to be better tolerated leading to enhanced compliance.
Table 25–4. Commonly used antipsychotic medications (listed in alphabetical order).

Table 25–5. Relative potency and side effects of antipsychotic medications (listed in alphabetical order).
The phenothiazines comprise the bulk of the currently used “typical” or first-generation antipsychotic medications. The only butyrophenone commonly used in psychiatry is haloperidol, which is different in structure but similar in action and side effects to the piperazine phenothiazines such as fluphenazine, perphenazine, and trifluoperazine. These medications and haloperidol (dopamine [D2] receptor blockers) have high potency and a paucity of autonomic side effects and act to markedly lower arousal levels.
Clozapine, the first “atypical” (novel) antipsychotic medication developed, has dopamine (D4) receptor-blocking activity as well as central serotonergic, histaminergic, and alpha-noradrenergic receptor-blocking activity. It is effective in the treatment of about 30% of psychoses resistant to other antipsychotic medications, and it may have specific efficacy in decreasing suicidality in patients with schizophrenia. Risperidone is an antipsychotic that blocks some serotonin receptors (5-HT2) and dopamine receptors (D2). Risperidone causes fewer extrapyramidal side effects than the typical antipsychotics at doses less than 6 mg. It appears to be as effective as haloperidol and possibly as effective as clozapine in treatment-resistant patients without necessitating weekly white cell counts, as required with clozapine therapy. Risperidone-induced hyperprolactinemia, even on low doses, has been reported, and that effect is thought to be more common with risperidone than with other atypical antipsychotics. Risperidone is available in a long-acting injectable preparation.
Olanzapine is a potent blocker of 5-HT2 and dopamine D1, D2, and D4 receptors. High doses of olanzapine (10–20 mg daily) appear to be more effective than lower doses. The medication is somewhat more effective than haloperidol in the treatment of negative symptoms, such as withdrawal, psychomotor retardation, and poor interpersonal relationships. It is available in an orally disintegrating form for patients who are unable to tolerate standard oral dosing and in an injectable form for the management of acute agitation associated with schizophrenia and bipolar disorder. Olanzapine is available in a long-term injectable preparation, but this formulation tends to be used less commonly than other depot formulations because some patients experience severe sedation and delirium, which occurs in about 0.5–1% of patients.
Quetiapine is an antipsychotic with greater 5-HT2 relative to D2 receptor blockade as well as a relatively high affinity for alpha-1- and alpha-2-adrenergic receptors. It appears to be as efficacious as haloperidol in treating positive and negative symptoms of schizophrenia, with fewer extrapyramidal side effects even at high doses.
Ziprasidone has both anti-dopamine receptor and anti-serotonin receptor effects, with good efficacy for both positive and negative symptoms of schizophrenia. Aripiprazole is a partial agonist at the dopamine D2 and serotonin 5-HT1 receptors and an antagonist at 5-HT2 receptors, and it is effective against positive and negative symptoms of schizophrenia. It functions as an antagonist or agonist, depending on the dopaminergic activity at the dopamine receptors. This may help decrease side effects. Aripiprazole is approved as an augmentation agent for treatment-resistant depression, even when psychosis is not present, and as a maintenance treatment for bipolar disorder. Aripiprazole is available as an acute injectable preparation as well as a long-term injectable preparation that is given once monthly in patients who are not able to adhere to daily oral dosing. Asenapine, approved for the treatment of schizophrenia and bipolar disorder (mixed or manic state), appears to be particularly helpful in treating negative symptoms of schizophrenia. Paliperidone, the active metabolite of risperidone, is available as a capsule and a monthly injection. Lurasidone is FDA-approved and has been shown to be effective in treating acute decompensation in patients with chronic schizophrenia. Cariprazine is a partial agonist of the D2 and D3 receptor and is approved by the FDA for the treatment of schizophrenia and bipolar disorder. Akathisia, weight gain, and insomnia are among the more commonly reported side effects with cariprazine. Because cariprazine is not a potent D2-antagonist, it is less likely to increase prolactin levels than most antipsychotics.
Beyond antipsychotics, there is early evidence that cannabidiol (CBD), at a dose of 1000 mg/day, added on to existing antipsychotic treatment, effectively improves psychotic symptoms in schizophrenia. This agent may represent a new class of treatment for psychotic disorders.
1. Clinical indications—The antipsychotics are used to treat all forms of the schizophrenias as well as drug-induced psychoses, psychotic depression, augmentation of unipolar depression, acute mania, and the prevention of mood cycles in bipolar disorder. They are also effective in Tourette syndrome and behavioral dyscontrol in autistic patients. While frequently used to treat agitation in dementia patients, no antipsychotic has been shown to be reliably effective in this population and may increase the risk of early mortality in elderly dementia patients. Antipsychotics quickly lower the arousal (activity) level and, perhaps indirectly, gradually improve socialization and thinking. The improvement rate for treating positive symptoms is about 80%. Patients whose behavioral symptoms worsen with use of antipsychotic medications may have an undiagnosed organic condition such as anticholinergic toxicity.
Symptoms that are ameliorated by these medications include hyperactivity, hostility, aggression, delusions, hallucinations, irritability, and poor sleep. Individuals with acute psychosis and good premorbid function respond quite well. The most common cause of failure in the treatment of acute psychosis is inadequate dosage, and the most common cause of relapse is noncompliance.
Although first-generation antipsychotics are efficacious in the treatment of positive symptoms of schizophrenia, such as hallucinations and delusions, second-generation antipsychotics are thought to have efficacy in reducing positive symptoms and some efficacy in treating negative symptoms. Antidepressant medications may be used in conjunction with antipsychotics if significant depression is present. Resistant cases may require concomitant use of lithium, carbamazepine, or valproic acid. The addition of a benzodiazepine medication to the antipsychotic regimen may prove helpful in treating the agitated or catatonic psychotic patient who has not responded to antipsychotics alone—lorazepam, 1–2 mg orally, can produce a rapid resolution of catatonic symptoms and may allow maintenance with a lower antipsychotic dose. Electroconvulsive therapy (ECT) has also been effective in treating catatonia and in treating schizophrenia when used in combination with medications.
2. Dosage forms and patterns—The dosage range is quite broad (Table 25–4). For example, risperidone can be effective for some patients with psychotic features at 0.25–1 mg orally at bedtime, whereas up to 6 mg/day may be used in a young patient with acute schizophrenia. In an acutely distressed, psychotic patient one might use haloperidol, 10 mg intramuscularly, which is absorbed rapidly and achieves an initial tenfold plasma level advantage over equal oral doses. Psychomotor agitation, racing thoughts, and general arousal are quickly reduced. The dose can be repeated every 3–4 hours; when the patient is less symptomatic, oral doses can replace parenteral administration in most cases. In the elderly, both atypical (eg, risperidone 0.25 mg–0.5 mg daily or olanzapine 1.25 mg daily) and typical (eg, haloperidol 0.5 mg daily or perphenazine 2 mg daily) antipsychotics, often used effectively in small doses for behavioral control, have been linked to premature death in some cases.
Absorption of oral medications may be increased or decreased by concomitant administration of other medications (eg, antacids tend to decrease the absorption of antidepressants). Previous gastrointestinal surgery may alter pH, motility, and surface areas available for drug absorption. There are racial genetic-based enzyme differences in metabolizing the antipsychotic medications—eg, many people of Asian descent require only about half the usual dosage. Bioavailability is influenced by other factors such as smoking or hepatic microsomal enzyme stimulation with alcohol or barbiturates and enzyme-altering medications such as carbamazepine or methylphenidate. Antipsychotic plasma drug level determinations are not currently of major clinical assistance.
Divided daily doses are not necessary after a maintenance dose has been established, and most patients can then be maintained on a single daily dose, usually taken at bedtime. This is particularly appropriate in a case where the sedative effect of the medication is desired for nighttime sleep, and undesirable sedative effects can be avoided during the day. First-episode patients especially should be tapered off medications after about 6 months of stability and carefully monitored; their rate of relapse is lower than that of multiple-episode patients.
Psychiatric patients—particularly paranoid individuals— often neglect to take their medication. In these cases and in patients who do not respond to oral medication, the enanthate and decanoate (the latter is slightly longer-lasting and has fewer extrapyramidal side effects) forms of fluphenazine or the decanoate form of haloperidol may be given by deep subcutaneous injection or intramuscularly to achieve an effect that will usually last 7–28 days. A patient who cannot be depended on to take oral medication (or who overdoses on minimal provocation) will generally agree to come to the clinician’s office for a “shot.” The usual dose of the fluphenazine long-acting preparations is 25 mg every 2 weeks. Dosage and frequency of administration vary from about 12.5 mg monthly to 100 mg weekly. Use the smallest effective amount as infrequently as possible. A monthly injection of 25 mg of fluphenazine decanoate is equivalent to about 15–20 mg of oral fluphenazine daily. Risperidone was the first atypical antipsychotic available in a long-acting injectable form (25–50 mg intramuscularly every 2 weeks). Concomitant use of a benzodiazepine (eg, lorazepam, 2 mg orally twice daily) may permit reduction of the required dosage of oral or parenteral antipsychotic medication. Long-acting injectables are now available for risperidone, paliperidone, aripiprazole, and olanzapine.
Intravenous haloperidol, the antipsychotic most commonly used by this route, is often used in critical care units in the management of agitated, delirious patients. Intravenous haloperidol should be given no faster than 1 mg/min to reduce cardiovascular side effects, such as torsades de pointes. Current practice indicates that ECG monitoring should be used whenever haloperidol is being administered intravenously.
Some antipsychotic agents are available for intranasal administration. The intranasal form of loxapine has a more rapid onset of action for the treatment of agitation (about 10 minutes) than either intramuscular or oral antipsychotic agents. Also, intranasal administration tends to be less traumatic to patients than getting an injection. However, intranasal loxapine requires the cooperation of the patient and is more expensive than generic antipsychotic injectable preparations.
There have been investigations for novel compounds involving other therapeutics targets such as the glutamate system as well as the inflammatory cascade.
3. Side effects—For both typical and atypical antipsychotic agents, a range of side effects has been reported. The most common anticholinergic side effects include dry mouth (which can lead to ingestion of caloric liquids and weight gain or hyponatremia), blurred near vision, urinary retention (particularly in elderly men with enlarged prostates), delayed gastric emptying, esophageal reflux, ileus, delirium, and precipitation of acute glaucoma in patients with narrow anterior chamber angles. Other autonomic effects include orthostatic hypotension and sexual dysfunction—problems in achieving erection, ejaculation (including retrograde ejaculation), and orgasm in men (approximately 50% of cases) and women (approximately 30%). Delay in achieving orgasm is often a factor in medication noncompliance. Electrocardiographic changes occur frequently, but clinically significant arrhythmias are much less common. Elderly patients and those with preexisting cardiac disease are at greater risk. The most frequently seen electrocardiographic changes include diminution of the T wave amplitude, appearance of prominent U waves, depression of the ST segment, and prolongation of the QT interval (Table 25–6). Ziprasidone can produce QTc prolongation. A pretreatment ECG is indicated for patients at risk for cardiac sequelae (including patients taking other medications that might prolong the QTc interval). In some critical care patients, torsades de pointes has been associated with the use of high-dose intravenous haloperidol (usually greater than 30 mg/24 h).
Table 25–6. Adverse factors associated with atypical antipsychotic medications (listed in alphabetical order).
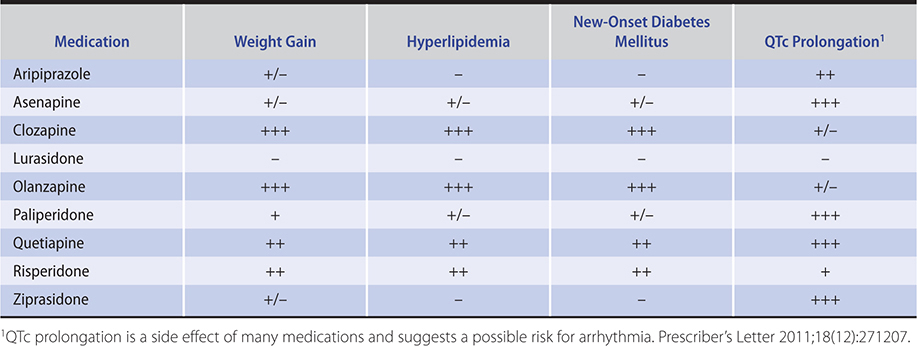
Associations have been suggested between the atypical antipsychotics and new-onset diabetes, hyperlipidemia, and weight gain (Table 25–6). The FDA has particularly noted the risk of hyperglycemia and new-onset diabetes in this class of medication that is not related to weight gain. The risk of diabetes mellitus is increased in patients taking clozapine and olanzapine. Monitoring of weight, fasting blood sugar, and lipids prior to initiation of treatment and at regular intervals thereafter is an important part of medication monitoring. The addition of metformin to olanzapine may improve drug-induced weight gain in patients with drug-naïve, first-episode schizophrenia. Antipsychotic medications in general may have metabolic and endocrine effects, including weight gain, hyperglycemia, impaired temperature regulation in hot weather, and water intoxication, which may be due to inappropriate ADH secretion. Lactation and menstrual irregularities are common (antipsychotic medications should be avoided, if possible, in breast cancer patients because of potential trophic effects of elevated prolactin levels on the breast). Both antipsychotic and antidepressant medications inhibit sperm motility. Bone marrow depression and cholestatic jaundice occur rarely; these are hypersensitivity reactions, and they usually appear in the first 2 months of treatment. They subside on discontinuance of the medication. There is cross-sensitivity among all of the phenothiazines, and a medication from a different group should be used when allergic reactions occur.
Clozapine is associated with a 1.6% risk of agranulocytosis (higher in persons of Ashkenazi Jewish ancestry), and its use must be strictly monitored with weekly blood counts during the first 6 months of treatment, with monitoring every other week thereafter. The risk of developing agranulocytosis is approximately 2.5 times higher in patients with a polymorphism for HLADQB1 gene. Thus, this genetic test may be worthwhile to perform before initiating clozapine. Discontinuation of the medication requires weekly monitoring of the white blood cell count for 1 month. Clozapine has been associated with fatal myocarditis and is contraindicated in patients with severe heart disease. In addition, clozapine lowers the seizure threshold and has many side effects, including sedation, hypotension, increased liver biochemical levels, hypersalivation, respiratory arrest, weight gain, and changes in both the ECG and the electroencephalogram. Notably, adynamic ileus is a rare side effect of clozapine that can be fatal, and patients should be closely monitored and treated quickly and preemptively for constipation.
Photosensitivity, retinopathy, and hyperpigmentation are associated with use of fairly high dosages of chlorpromazine. The appearance of particulate melanin deposits in the lens of the eye is related to the total dose given, and patients on long-term medication should have periodic eye examinations. Teratogenicity has not been causally related to these medications, but prudence is indicated particularly in the first trimester of pregnancy. The seizure threshold is lowered, but it is safe to use these medications in epileptics who take anticonvulsants.
The neuroleptic malignant syndrome (NMS) is a catatonia-like state manifested by extrapyramidal signs, blood pressure changes, altered consciousness, and hyperpyrexia; it is an uncommon but serious complication of antipsychotic treatment. Muscle rigidity, involuntary movements, confusion, dysarthria, and dysphagia are accompanied by pallor, cardiovascular instability, fever, pulmonary congestion, and diaphoresis and may result in stupor, coma, and death. The cause may be related to a number of factors, including poor dosage control of antipsychotic medication, affective illness, decreased serum iron, dehydration, and increased sensitivity of dopamine receptor sites. Lithium in combination with an antipsychotic medication may increase vulnerability, which is already increased in patients with an affective disorder. In most cases, the symptoms develop within the first 2 weeks of antipsychotic drug treatment. The syndrome may occur with small doses of the medications. Intramuscular administration is a risk factor. Elevated creatine kinase and leukocytosis with a shift to the left are present early in about half of cases. Treatment includes controlling fever and providing fluid support. Dopamine agonists such as bromocriptine, 2.5–10 mg orally three times a day, and amantadine, 100–200 mg orally twice a day, have also been useful. Dantrolene, 50 mg intravenously as needed, is used to alleviate rigidity (do not exceed 10 mg/kg/day due to hepatotoxicity risk). There is ongoing controversy about the efficacy of these three agents as well as the use of calcium channel blockers and benzodiazepines. ECT has been used effectively in resistant cases. Clozapine has been used with relative safety and fair success as an antipsychotic medication for patients who have had NMS.
Akathisia is the most common (about 20%) extrapyramidal symptom. It usually occurs early in treatment (but may persist after antipsychotics are discontinued) and is frequently mistaken for anxiety or exacerbation of psychosis. It is characterized by a subjective desire to be in constant motion followed by an inability to sit or stand still and consequent pacing. It may induce suicidality or feelings of fright, rage, terror, or sexual torment. Insomnia is often present. It is crucial to educate patients in advance about these potential side effects so that the patients do not misinterpret them as signs of increased illness. In all cases, reevaluate the dosage requirement or the type of antipsychotic medication. One should inquire also about cigarette smoking, which in women has been associated with an increased incidence of akathisia. Antiparkinsonism medications (such as trihexyphenidyl, 2–5 mg orally three times daily) may be helpful, but first-line treatment often includes a benzodiazepine (such as clonazepam 0.5–1 mg orally three times daily). In resistant cases, symptoms may be alleviated by propranolol, 30–80 mg/day orally, diazepam, 5 mg orally three times daily, or amantadine, 100 mg orally three times daily.
Acute dystonias usually occur early, although a late (tardive) occurrence is reported in patients (mostly men after several years of therapy) who previously had early severe dystonic reactions and a mood disorder. Younger patients are at higher risk for acute dystonias. The most common signs are bizarre muscle spasms of the head, neck, and tongue. Frequently present are torticollis, oculogyric crises, swallowing or chewing difficulties, and masseter spasms. Laryngospasm is particularly dangerous. Back, arm, or leg muscle spasms are occasionally reported. Diphenhydramine, 50 mg intramuscularly, is effective for the acute crisis; one should then give benztropine mesylate, 2 mg orally twice daily, for several weeks, and then discontinue gradually, since few of the extrapyramidal symptoms require long-term use of the antiparkinsonism medications (all of which are about equally efficacious—though trihexyphenidyl tends to be mildly stimulating and benztropine mildly sedating).
Drug-induced parkinsonism is indistinguishable from idiopathic parkinsonism, but it is reversible, occurs later in treatment than the preceding extrapyramidal symptoms, and in some cases appears after antipsychotic withdrawal. The condition includes the typical signs of apathy and reduction of facial and arm movements (akinesia, which can mimic depression), festinating gait, rigidity, loss of postural reflexes, and pill-rolling tremor. Patients with AIDS seem particularly vulnerable to extrapyramidal side effects. High-potency antipsychotics often require antiparkinsonism medications. The antipsychotic dosage should be reduced, and immediate relief can be achieved with antiparkinsonism medications in the same dosages as above. After 4–6 weeks, these antiparkinsonism medications can often be discontinued with no recurrent symptoms. In any of the extrapyramidal symptoms, amantadine, 100–400 mg orally daily, may be used instead of the antiparkinsonism medications. Antipsychotic-induced catatonia is similar to catatonic stupor with rigidity, drooling, urinary incontinence, and cogwheeling. It usually responds slowly to withdrawal of the offending medication and use of antiparkinsonism agents.
Tardive dyskinesia is a syndrome of abnormal involuntary stereotyped movements of the face, mouth, tongue, trunk, and limbs that may occur after months or (usually) years of treatment with antipsychotic agents. The syndrome affects 20–35% of patients who have undergone long-term antipsychotic therapy. Predisposing factors include older age, many years of treatment, cigarette smoking, and diabetes mellitus. Pineal calcification is higher in this condition by a margin of 3:1. There are no clear-cut differences among the antipsychotic medications in the development of tardive dyskinesia. (Although the atypical antipsychotics appear to offer a lower risk of tardive dyskinesia, long-term effects have not been investigated.) However, clozapine is unique in that it has been found to treat antipsychotic-induced tardive dyskinesia. Early manifestations of tardive dyskinesia include fine worm-like movements of the tongue at rest, difficulty in sticking out the tongue, facial tics, increased blink frequency, or jaw movements of recent onset. Later manifestations may include bucco-linguo-masticatory movements, lip smacking, chewing motions, mouth opening and closing, disturbed gag reflex, puffing of the cheeks, disrupted speech, respiratory distress, or choreoathetoid movements of the extremities (the last being more prevalent in younger patients). The symptoms do not necessarily worsen and in rare cases may lessen even though antipsychotic medications are continued. The dyskinesias do not occur during sleep and can be voluntarily suppressed for short periods. Stress and movements in other parts of the body will often aggravate the condition.
Early signs of dyskinesia must be differentiated from those reversible signs produced by ill-fitting dentures or nonantipsychotic medications such as levodopa, TCAs, antiparkinsonism agents, anticonvulsants, and antihistamines. Other neurologic conditions such as Huntington chorea can be differentiated by history and examination.
The emphasis should be on prevention of side effects. Use the least amount of antipsychotic medication necessary to mute the psychotic symptoms. Detect early manifestations of dyskinesias. When these occur, stop anticholinergic medications and gradually discontinue antipsychotic medications, if clinically feasible. Weight loss and cachexia sometimes appear on withdrawal of antipsychotics. In an indeterminate number of cases, the dyskinesias will remit. Keep the patient off the medications until reemergent psychotic symptoms dictate their resumption, at which point they are restarted in low doses and gradually increased until there is clinical improvement. If antipsychotic medications are restarted, clozapine and olanzapine appear to offer less risk of recurrence. The use of adjunctive agents such as benzodiazepines or lithium may help directly or indirectly by allowing control of psychotic symptoms with a low dosage of antipsychotics. If the dyskinesic syndrome recurs and it is necessary to continue antipsychotic medications to control psychotic symptoms, informed consent should be obtained. Benzodiazepines, buspirone (in doses of 15–60 mg/day orally), phosphatidylcholine, clonidine, calcium channel blockers, vitamin E, omega-3 fatty acids, and propranolol all have had limited usefulness in treating the dyskinetic side effects.
B. Social
Environmental considerations are most important in the individual with a chronic illness, who usually has a history of repeated hospitalizations, a continued low level of functioning, and symptoms that never completely remit. Family rejection and work failure are common. In these cases, board and care homes staffed by personnel experienced in caring for psychiatric patients are most important. There is frequently an inverse relationship between stability of the living situation and the amounts of required antipsychotic medications, since the most salutary environment is one that reduces stimuli. Nonresidential self-help groups such as Recovery, Inc., should be utilized whenever possible. They provide a setting for sharing, learning, and mutual support and are frequently the only social involvement with which this type of patient is comfortable. Vocational rehabilitation and work agencies (eg, Goodwill Industries, Inc.) provide assessment, training, and job opportunities at a level commensurate with the person’s clinical condition.
C. Psychological
The need for psychotherapy varies markedly depending on the patient’s current status and history. In a person with a single psychotic episode and a previously good level of adjustment, supportive psychotherapy may help the patient reintegrate the experience, gain some insight into antecedent problems, and become a more self-observant individual who can recognize early signs of stress. Research suggests that cognitive behavioral therapy—in conjunction with medication management—has efficacy in the treatment of symptoms of schizophrenia. Cognitive behavioral therapy for schizophrenia involves helping the individual challenge psychotic thinking and alters response to hallucinations. Similarly, a form of psychotherapy called acceptance and commitment therapy has shown value in helping prevent hospitalizations in schizophrenia. Cognitive remediation therapy is another approach to treatment that may help patients with schizophrenia become better able to focus their disorganized thinking. Family therapy may also help alleviate the patient’s stress and to assist relatives in coping with the patient.
D. Behavioral
Behavioral techniques (see above) are most frequently used in therapeutic settings such as day treatment centers, but they can also be incorporated into family situations or any therapeutic setting. Many behavioral techniques (eg, positive reinforcement—whether it be a word of praise or an approving nod—after some positive behavior) can be a powerful instrument for helping a person learn behaviors that will facilitate social acceptance. Music from portable digital players or smartphones with earphones is one of many ways to divert the patient’s attention from auditory hallucinations.
 Prognosis
Prognosis
For most patients with any psychosis, the prognosis is good for alleviation of positive symptoms such as hallucinations or delusions treated with medication. Negative symptoms such as diminished affect and sociability are much more difficult to treat but appear mildly responsive to atypical antipsychotics. Cognitive deficits, such as the executive dysfunction that is common to schizophrenia, also do not appear as responsive to antipsychotics as do positive symptoms. Unfortunately, both negative symptoms and cognitive deficits appear to contribute more to long-term disability than do positive symptoms. Unavailability of structured work situations and lack of family therapy or access to other social support are two other reasons why the prognosis is so guarded in such a large percentage of patients.
Huhn M et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019 Sep 14;394(10202):939–51. Erratum in: Lancet. 2019 Sep 14;394(10202):918. [PMID: 31303314]
McGuire P et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018 Mar 1;175(3):225–31. [PMID: 29241357]
Tiihonen J et al. Real-world effectiveness of antipsychotic treatments in a nationwide cohort of 29 823 patients with schizophrenia. JAMA Psychiatry. 2017 Jul 1;74(7):686–93. [PMID: 28593216]
Winton-Brown TT et al. Five-fold increased risk of relapse following breaks in antipsychotic treatment of first episode psychosis. Schizophr Res. 2017 Jan;179:50–6. [PMID: 27745754]
MOOD DISORDERS (Depression & Mania)
ESSENTIALS OF DIAGNOSIS

Present in most depressions
 Mood varies from mild sadness to intense despondency and feelings of guilt, worthlessness, and hopelessness.
Mood varies from mild sadness to intense despondency and feelings of guilt, worthlessness, and hopelessness.
 Difficulty in thinking, including inability to concentrate, ruminations, and lack of decisiveness.
Difficulty in thinking, including inability to concentrate, ruminations, and lack of decisiveness.
 Loss of interest, with diminished involvement in work and recreation.
Loss of interest, with diminished involvement in work and recreation.
 Somatic complaints such as disrupted, lessened, or excessive sleep; loss of energy; change in appetite; decreased sexual drive.
Somatic complaints such as disrupted, lessened, or excessive sleep; loss of energy; change in appetite; decreased sexual drive.
Present in some severe depressions
 Psychomotor retardation or agitation.
Psychomotor retardation or agitation.
 Delusions of a somatic or persecutory nature.
Delusions of a somatic or persecutory nature.
 Withdrawal from activities.
Withdrawal from activities.
 Physical symptoms of major severity, eg, anorexia, insomnia, reduced sexual drive, weight loss, and various somatic complaints.
Physical symptoms of major severity, eg, anorexia, insomnia, reduced sexual drive, weight loss, and various somatic complaints.
 Suicidal ideation.
Suicidal ideation.
Possible symptoms in mania
 Mood ranging from euphoria to irritability.
Mood ranging from euphoria to irritability.
 Sleep disruption.
Sleep disruption.
 Hyperactivity.
Hyperactivity.
 Racing thoughts.
Racing thoughts.
 Grandiosity or extreme overconfidence.
Grandiosity or extreme overconfidence.
 Variable psychotic symptoms.
Variable psychotic symptoms.
 General Considerations
General Considerations
Depression is extremely common, with up to 30% of primary care patients having depressive symptoms. Depression may be the final expression of (1) genetic factors (neurotransmitter dysfunction), (2) developmental problems (personality problems, childhood events), or (3) psychosocial stresses (divorce, unemployment). It frequently presents in the form of somatic complaints with negative medical workups. Although sadness and grief are normal responses to loss, depression is not. Patients experiencing normal grief tend to produce sympathy and sadness in the clinician caregiver; depression often produces frustration and irritation in the clinician. Grief is usually accompanied by intact self-esteem, whereas depression is marked by a sense of guilt and worthlessness.
Mania is often combined with depression and may occur alone, together with depression in a mixed episode, or in cyclic fashion with depression.
 Clinical Findings
Clinical Findings
In general, there are four major types of depression, with similar symptoms in each group.
A. Adjustment Disorder with Depressed Mood
Depression may occur in reaction to some identifiable stressor or adverse life situation, usually loss of a person by death (grief reaction), divorce, etc; financial reversal (crisis); or loss of an established role, such as being needed. Anger is frequently associated with the loss, and this in turn often produces a feeling of guilt. The disorder occurs within 3 months of the stressor and causes significant impairment in social or occupational functioning. The symptoms range from mild sadness, anxiety, irritability, worry, and lack of concentration, discouragement, and somatic complaints to the more severe symptoms of frank depression. When the full criteria for major depressive disorder are present, however, then that diagnosis should be made and treatment instituted even when there is a known stressor. The presence of a stressor is not the determining diagnostic driver, it is the resultant syndromal complex. One should not neglect treatment for major depression simply because it may appear to be an understandable reaction to a particular stress or difficulty.
B. Depressive Disorders
The subclassifications include major depressive disorder and dysthymia.
1. Major depressive disorder—A major depressive disorder consists of a syndrome of mood, physical and cognitive symptoms that occurs at any time of life. Many consider a physiologic or metabolic aberration to be causative. Complaints vary widely but most frequently include a loss of interest and pleasure (anhedonia), withdrawal from activities, and feelings of guilt. Also included are inability to concentrate, some cognitive dysfunction, anxiety, chronic fatigue, feelings of worthlessness, somatic complaints (unexplained somatic complaints frequently indicate depression), loss of sexual drive, and thoughts of death. Unemployment has been associated with increase in depression risk. Diurnal variation with improvement as the day progresses is common. Vegetative signs that frequently occur are insomnia, anorexia with weight loss, and constipation. Occasionally, severe agitation and psychotic ideation are present. Psychotic major depression occurs up to 14% of all patients with major depression and 25% of patients who are hospitalized with depression. Psychotic symptoms (delusions, paranoia) are more common in depressed persons who are older than 50 years. Paranoid symptoms may range from general suspiciousness to ideas of reference with delusions. The somatic delusions frequently revolve around feelings of impending annihilation or somatic concerns (eg, that the body is rotting away with cancer). Hallucinations are less common than unusual beliefs and tend not to occur independent of delusions.
In addition to psychotic major depression, other subcategories include major depression with atypical features that is characterized by hypersomnia, overeating, lethargy, and mood reactivity in which the mood brightens in response to positive events or news. Melancholic major depression is characterized by a lack of mood reactivity seen in atypical depression, the presence of a prominent anhedonia, and more severe vegetative symptoms. Major depression with a seasonal onset (seasonal affective disorder) is a dysfunction of circadian rhythms that occurs more commonly in the fall and winter months and is believed to be due to decreased exposure to full-spectrum light. Common symptoms include carbohydrate craving, lethargy, hyperphagia, and hypersomnia. Major depression with peripartum onset occurs during pregnancy or starts up to 4 weeks after delivery.
Half of depressions associated with the peripartum period start during pregnancy. Most women (up to 80%) experience some mild letdown of mood in the postpartum period. For some of these (10–15%), the symptoms are more severe and similar to those usually seen in serious depression, with an increased emphasis on concerns related to the baby (obsessive thoughts about harming it or inability to care for it). When psychotic symptoms occur, there is frequently associated sleep deprivation, volatility of behavior, and manic-like symptoms. Postpartum psychosis is much less common (less than 2%), often occurs within the first 2 weeks, and requires early and aggressive management. Biologic vulnerability with hormonal changes and psychosocial stressors all play a role. The chances of a second episode are about 25% and may be reduced with prophylactic treatment.
2. Persistent depressive disorder (dysthymia)—Dysthymia is a chronic depressive disturbance. Sadness, loss of interest, and withdrawal from activities over a period of 2 or more years with a relatively persistent course are necessary for this diagnosis. Generally, the symptoms are milder but longer-lasting than those in a major depressive episode.
3. Premenstrual dysphoric disorder—Depressive symptoms occur during the late luteal phase (last 2 weeks) of the menstrual cycle. (See also Chapter 18.)
C. Bipolar Disorder
Bipolar disorder consists of episodic mood shifts into mania, major depression, hypomania, and mixed mood states. The ability of bipolar disorder to mimic aspects of many other coincident major mental health disorders and a high comorbidity with substance abuse can make the initial diagnosis of bipolar disorder difficult. Bipolar I is diagnosed when an individual has manic episodes. For individuals who experience hypomanic episodes without frank mania, the diagnosis is bipolar II.
1. Mania—A manic episode is a mood state characterized by elation with hyperactivity, overinvolvement in life activities, increased irritability, flight of ideas, easy distractibility, and little need for sleep. The overenthusiastic quality of the mood and the expansive behavior initially attract others, but the irritability, mood lability with swings into depression, aggressive behavior, and grandiosity usually lead to marked interpersonal difficulties. Activities may occur that are later regretted, eg, excessive spending, resignation from a job, a hasty marriage, sexual acting out, and exhibitionistic behavior, with alienation of friends and family. Atypical manic episodes can include gross delusions, paranoid ideation of severe proportions, and auditory hallucinations usually related to some grandiose perception. The episodes begin abruptly (sometimes precipitated by life stresses) and may last from several days to months. Generally, the manic episodes are of shorter duration than the depressive episodes. In almost all cases, the manic episode is part of a broader bipolar disorder. Patients with four or more discrete episodes of a mood disturbance in 1 year have “rapid cycling.” (Substance abuse, particularly cocaine, can mimic rapid cycling.) These patients have a higher incidence of hypothyroidism. Patients with mania differ from patients with schizophrenia in that the former use more effective interpersonal maneuvers, are more sensitive to the social maneuvers of others, and are more able to utilize weakness and vulnerability in others to their own advantage. Creativity has been positively correlated with mood disorders, but the best work done is between episodes of mania and depression.
2. Cyclothymic disorder—This is a chronic mood disturbance with episodes of subsyndromal depression and hypomania. The symptoms must have at least a 2-year duration and are milder than those that occur in depressive or manic episodes. Occasionally, the symptoms will escalate into a full-blown manic or depressive episode, in which case reclassification as bipolar I or II would be warranted.
D. Mood Disorders Secondary to Illness and Medications
Any illness, severe or mild, can cause significant depression. Conditions such as rheumatoid arthritis, multiple sclerosis, stroke, and chronic heart disease are particularly likely to be associated with depression, as are other chronic illnesses. Depression is common in cancer, as well, with a particularly high degree of comorbidity in pancreatic cancer. Hormonal variations clearly play a role in some depressions. Varying degrees of depression occur at various times in schizophrenic disorders, central nervous system disease, and organic mental states. Alcohol dependency frequently coexists with serious depression.
The classic model of drug-induced depression occurred with the use of reserpine, both in clinical settings and as a pharmacologic probe in research settings. Corticosteroids and oral contraceptives are commonly associated with mood changes such as depression and hypomania. Antihypertensive medications such as methyldopa, guanethidine, and clonidine have been associated with the development of depressive syndromes, as have digitalis and antiparkinsonism medications (eg, levodopa). Retinoids have been associated with depression, and interferon is strongly associated with depressed mood and fatigue as a side effect; consultation with a psychiatrist prior to prescribing these agents is indicated in cases where there is a history of depression. Overall, the literature has not shown an association between beta-blocker use and depression. Infrequently, disulfiram and anticholinesterase medications may be associated with symptoms of depression. Stimulant use results in a depressive syndrome when the drug is withdrawn. Alcohol, sedatives, and opioids are depressants and, paradoxically, are often used in self-treatment of depression. Corticosteroids may be associated with development of hypomania.
 Differential Diagnosis
Differential Diagnosis
Since depression may be a part of any illness—either reactively or as a secondary symptom—careful attention must be given to personal life adjustment problems and the role of medications (eg, reserpine, corticosteroids, levodopa). Schizophrenia, partial complex seizures, organic brain syndromes, panic disorders, and anxiety disorders must be differentiated. Thyroid dysfunction and other endocrinopathies should be ruled out. Malignancies, including central and gastrointestinal tumors are sometimes associated with depressive symptoms and may antecede the diagnosis of tumor. Strokes, particularly dominant hemisphere lesions, can occasionally present with a syndrome that looks like major depression. Medication-induced depressive symptoms are also quite common.
 Complications
Complications
The most important complication is suicide, which often includes some elements of aggression. Suicide rates in the general population vary from 9 per 100,000 in Spain to 15 per 100,000 in the United States to 31 per 100,000 in Russia. In individuals hospitalized for depression, the lifetime risk rises to 4–6%. In patients with bipolar I disorder, the risk is higher. Men over the age of 50 are more likely to complete a suicide because of their tendency to attempt suicide with more violent means, particularly guns. On the other hand, women make more attempts but are less likely to complete a suicide. An increased suicide rate is being observed in the younger population, aged 15–35. Patients with cancer, respiratory illnesses, AIDS, and those being maintained on hemodialysis have higher suicide rates. Alcohol use is a significant factor in many suicide attempts.
There are several groups of people who make suicide attempts. One group includes those individuals with acute situational problems. These individuals may be acutely distressed by a recent breakup in a relationship or another type of disappointment. This group also includes those who may not be diagnosed as having depression, but who are overwhelmed by a stressful situation often with an aspect of public humiliation (eg, victims of cyber-bullying). A suicide attempt in such cases may be an impulsive or aggressive act not associated with significant depression.
Another high-risk group includes individuals with severe depression. Severe depression may be due to conditions such as medical illness (eg, AIDS, whose victims have a suicide rate over 20 times that of the general population) or comorbid psychiatric disorders (eg, panic disorders). Anxiety, panic, and fear are major findings in suicidal behavior. A patient may seem to make a dramatic improvement, but the lifting of depression may be due to the patient’s decision to commit suicide. Another high-risk group are individuals with psychotic illness who tend not to verbalize their concerns and are often successful in their suicide attempt, although they make up only a small percentage of the total.
Suicide is 10 times more prevalent in patients with schizophrenia than in the general population, and jumping from bridges is a more common means of attempted suicide by patients with schizophrenia than by others. In one study of 100 people who jumped from bridges, 47% had schizophrenia.
The immediate goal of psychiatric evaluation is to assess the current suicidal risk and the need for hospitalization versus outpatient management. A useful question is to ask the person how many hours per day he or she thinks about suicide. If it is more than 1 hour, the individual is at high risk. Further assessing the risk by inquiring about intent, plans, means, and suicide-inhibiting factors (eg, strong ties to children or the church) is essential. Alcohol, hopelessness, delusional thoughts, and complete or nearly complete loss of interest in life or ability to experience pleasure are all positively correlated with suicide attempts. Other risk factors are previous attempts, a family history of suicide, medical or psychiatric illness (eg, anxiety, depression, psychosis), male sex, older age, contemplation of violent methods, a humiliating social stressor, and drug use (including long-term sedative or alcohol use), which contributes to impulsiveness or mood swings. Successful treatment of the patient at risk for suicide cannot be achieved if the patient continues to abuse drugs. An attempt is less likely to be suicidal, for example, if small amounts of poison or medication were ingested or scratching of wrists was superficial, if the act was performed near others or with early notification of others, or if the attempt was arranged so that early detection would be anticipated.
The patient’s current mood status is best evaluated by direct evaluation of plans and concerns about the future, personal reactions to the attempt, and thoughts about the reactions of others. Measurement of mood is often facilitated by using a standardized instrument such as the Hamilton or Montgomery-Asberg clinician-administered rating scales or the self-administered Quick Inventory of Depressive Symptomatology (QIDS-SR 16). Scales allow for initial assessment as well as ongoing treatment tracking. Suicide risk can be specifically assessed using an instrument such as the Columbia-Suicide Severity Risk Scale. The patient’s immediate resources should also be assessed—people who can be significantly involved (most important), family support, job situation, financial resources, etc.
If hospitalization is not indicated after a suicide attempt, the clinician must formulate and institute a treatment plan or make an adequate referral. (The National Suicide Prevention Lifeline, 1-800-273-8255, may be of assistance.) Medication should be dispensed in small amounts to at-risk patients. Although TCAs and SSRIs are associated with an equal incidence of suicide attempts, the risk of a completed suicide is much higher with TCA overdose. Guns and medications should be removed from the patient’s household. Driving should be interdicted until the patient improves. The problem is often worsened by the long-term complications of the suicide attempt, eg, brain damage due to hypoxia, peripheral neuropathies caused by staying for long periods in one position causing nerve compressions, and medical or surgical problems such as esophageal strictures and tendon dysfunctions.
 Treatment of Depression
Treatment of Depression
A. Medical
Milder forms of depression usually do not require medication therapy and can be managed by psychotherapy and the passage of time. In severe cases—particularly when vegetative signs are significant and symptoms have persisted for more than a few weeks—antidepressant medication therapy is often effective. Medication therapy is also suggested by a family history of major depression in first-degree relatives or a past history of prior episodes.
The antidepressant medications may be classified into four groups: (1) the newer antidepressants, including the SSRIs, SNRIs, and bupropion, vilazodone, vortioxetine, and mirtazapine, (2) the TCAs and clinically similar medications, (3) the MAO inhibitors (Table 25–7), and (4) stimulants. ECT and repetitive transcranial magnetic stimulation are procedural treatments for depression. These modalities are described in greater detail below.
Table 25–7. Commonly used antidepressant medications (listed in alphabetical order within classes).

Hospitalization is necessary if suicide is a major consideration or if complex treatment modalities are required.
Medication selection is influenced by the history of previous response or lack thereof if that information is available. There is mixed and inconclusive evidence about the utility and cost-effectiveness of genetic testing to choose an antidepressant treatment strategy. A positive family history of response to a particular medication may suggest that the patient will respond similarly. If no background information is available, a medication such as sertraline, 25 mg orally daily and increasing gradually up to 200 mg depending on response and side effects, or venlafaxine at 37.5 mg/day and titrated gradually as indicated to a maximum dose of 225 mg/day can be selected and a full trial instituted. The medication trial should be monitored for worsening mood or suicidal ideation with patient assessments every 1–2 weeks until week 6. The STAR*D trial suggests that if there is no response to the first medication, the best alternatives are to switch to a second agent that may be from the same or different class of antidepressant; another option if there is partial response is to try augmenting the first agent with bupropion (150–450 mg/day), lithium (eg, 300–900 mg/day orally), thyroid medication (eg, liothyronine, 25–50 mcg/day orally), or a second-generation antipsychotic (eg, aripiprazole [5–15 mg/day]). The Agency for Health Care Policy and Research has produced clinical practice guidelines that outline one algorithm of treatment decisions (Figure 25–2).
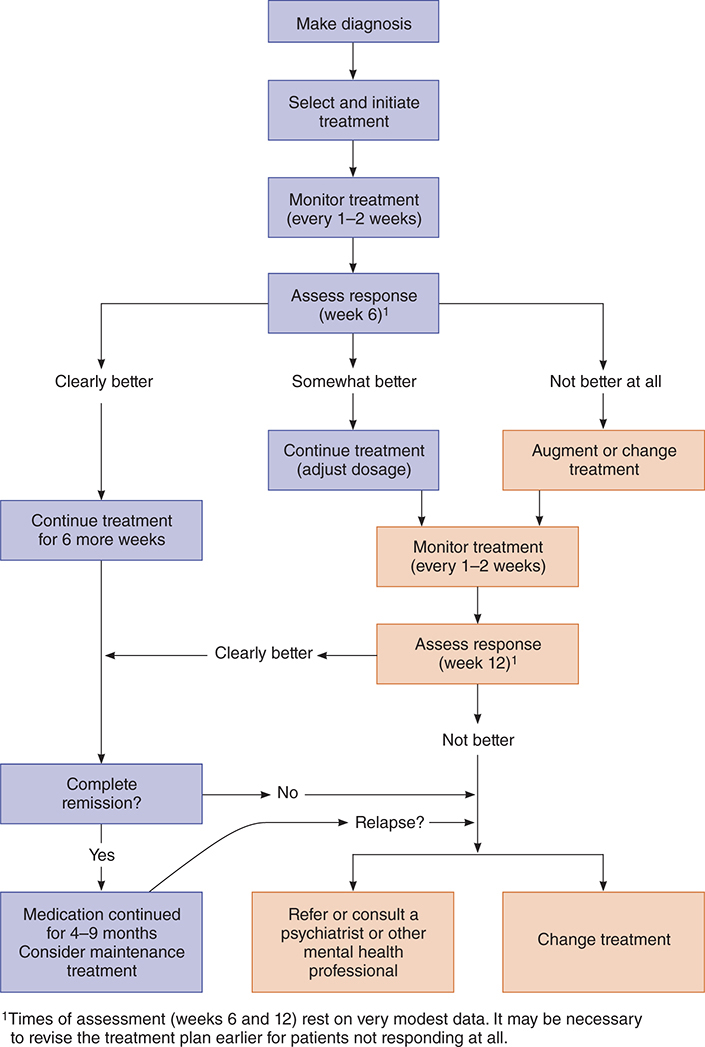
Figure 25–2. Overview of treatment for depression. (Reproduced from Agency for Health Care Policy and Research: Depression in Primary Care. Vol. 2: Treatment of Major Depression. United States Department of Health and Human Services, 1993.)
Cognitive issues such as concentration and memory problems are common to depression; the evidence shows that these issues sometimes persist even after depression has remitted, with a higher risk in those individuals who have had more depressive episodes.
Psychotic depression should be treated with a combination of an antipsychotic such as olanzapine and an antidepressant such as an SSRI at their usual doses. Mifepristone may have specific and early activity against psychotic depression. ECT is generally regarded as the single most effective treatment for psychotic depression, with remission rates between 60% and 90%.
Major depression with atypical features or seasonal onset can be treated with bupropion or an SSRI with good results. MAO inhibitors appear more effective than TCAs, and a MAO inhibitor may be used if more benign antidepressant strategies prove unsuccessful.
Melancholic depression may respond to ECT, TCAs, and SNRIs, which are preferable to SSRIs. However, SSRIs are often used in the treatment of melancholic depression and are effective in many cases.
Caution: Depressed patients often have suicidal thoughts, and the amount of medication dispensed should be appropriately controlled particularly if prescribing a MAO inhibitor, TCA, and to a lesser extent, venlafaxine. At the same time, adults with untreated depression are at higher risk for suicide than those who are treated sufficiently to reduce symptoms. It has been thought that in children and adolescent populations, antidepressants may be associated with some slightly increased risk of suicidality. One meta-analysis indicates that suicidality persists even after symptoms of depression are treated, suggesting other causes such as increased impulsivity among younger patients. After age 25, antidepressants may have neutral or possibly protective effects until age 65 years or older. The older TCAs have a narrow therapeutic index. One advantage of the newer medications is their wider margin of safety. Nonetheless, even with newer agents, because of the possibility of suicidality early in antidepressant treatment, close follow-up is indicated. In all cases of pharmacologic management of depressed states, caution is indicated until the risk of suicide is considered minimal.
1. SSRIs, SNRIs, and atypical antidepressants—SSRIs include fluoxetine, sertraline, paroxetine, fluvoxamine, citalopram and its enantiomer escitalopram (Table 25–7). The chief advantages of these agents are that they are generally well tolerated, the starting dose is typically a therapeutic dose for most patients, and they have much lower lethality in overdose compared to TCAs or MAO inhibitors. (Notably, citalopram carries a warning regarding QT prolongation in doses above 40 mg, and 20 mg is considered the maximum dose for patients older than 60 years. There is no similar FDA warning for escitalopram.) The SNRIs include venlafaxine, desvenlafaxine, duloxetine, milnacipran, and levomilnacipran. In addition to possessing the strong serotonin reuptake blocking properties of the SSRIs, the SNRIs are also norepinephrine reuptake blockers. The combined serotonergic-noradrenergic properties of these medications may provide benefits in pain conditions such as neuropathy and fibromyalgia as well as conditions such as stress incontinence. The atypical antidepressants are bupropion, nefazodone, trazodone, vilazodone, vortioxetine, and mirtazapine (Table 25–7). All of these antidepressants are effective in the treatment of depression, both typical and atypical. The SSRI medications have been effective in the treatment of panic disorder, bulimia, GAD, OCD, and PTSD.
Most of the medications in this group tend to be activating and are given in the morning so as not to interfere with sleep. Some patients, however, may have sedation, requiring that the medication be given at bedtime. This reaction occurs most commonly with paroxetine, fluvoxamine, and mirtazapine. The SSRIs can be given in once-daily dosage. Nefazodone and trazodone are usually given twice daily. Bupropion and venlafaxine are available in extended-release formulations and can be given once daily. There is usually some delay in response; fluoxetine, for example, requires 2–6 weeks to act in depression, 4–8 weeks to be effective in panic disorder, and 6–12 weeks in treatment of OCD. The starting dose (10 mg) is given for 1 week before increasing to the average daily oral dose of 20 mg for depression, while OCD may require up to 80 mg daily. Some patients, particularly the elderly, may tolerate and benefit from as little as 10 mg/day or every other day. The other SSRIs have shorter half-lives and a lesser effect on hepatic enzymes, which reduces their impact on the metabolism of other medications (thus not increasing significantly the serum concentrations of other medications as much as fluoxetine). The shorter half-lives also allow for more rapid clearing if adverse side effects appear. Venlafaxine appears to be more effective with doses greater than 200 mg/day orally, although some individuals respond to doses as low as 75 mg/day.
The side effects common to these medications are headache, nausea, tinnitus, insomnia, and nervousness. Akathisia has been common with the SSRIs; other extrapyramidal symptoms (eg, dystonias) have occurred infrequently but particularly in withdrawal states. Because SSRIs affect platelet serotonin levels, abnormal bleeding can occur. Sertraline and citalopram appear to be the safest agents in this class when used with warfarin. Sexual side effects of erectile dysfunction, retrograde ejaculation, and dysorgasmia are very common with the SSRIs. Oral phosphodiesterase-5 inhibitors (such as sildenafil, 25–50 mg; tadalafil, 5-20 mg; or vardenafil, 10–20 mg taken 1 hour prior to sexual activity) can improve erectile dysfunction in some patients and have been shown to improve other SSRI-induced sexual dysfunction in both men and women. Adjunctive bupropion (75–150 mg orally daily) may also enhance sexual arousal. Cyproheptadine, 4 mg orally prior to sexual activity, may be helpful in countering drug-induced anorgasmia but also is quite sedating and may counter the therapeutic benefits of SSRIs as well. Taking a “drug holiday,” ie, skipping a day of medication periodically when sexual activity is anticipated, can also decrease sexual side effects. The SSRIs are strong serotonin uptake blockers and may in high dosage or in combination with MAO inhibitors, including the antiparkinsonian drug selegiline, cause a “serotonin syndrome.” This syndrome is manifested by rigidity, hyperthermia, autonomic instability, myoclonus, confusion, delirium, and coma. This syndrome can be a particularly troublesome problem in the elderly. Research indicates that SSRIs are safer agents to use than TCAs in patients with cardiac disease; the SSRI sertraline is a safe and effective antidepressant treatment in patients with acute myocardial infarction or unstable angina.
Withdrawal symptoms, including dizziness, paresthesias, dysphoric mood, agitation, and a flu-like state, have been reported for the shorter-acting SSRIs and SNRIs but may occur with other classes including the TCAs and MAO inhibitors. These medications should be discontinued gradually over a period of weeks or months to reduce the risk of withdrawal phenomena.
Most studies show that SSRIs are not associated with birth defects. Paroxetine has some association with a fetal heart defect and should be avoided in favor of other SSRIs during pregnancy. Maternal major mood disorder in pregnancy by itself carries its own risks to the mother and fetus and has been linked to low birth weight and preterm delivery. Postpartum effects of prenatal depression have not been studied. The decision to use SSRIs and other psychotropic agents during pregnancy and postpartum must be a collaborative decision based on a thorough risk-benefit analysis for each individual.
Venlafaxine lacks significant anticholinergic side effects. Nausea, nervousness, and profuse sweating appear to be the major side effects. Venlafaxine appears to have few drug-drug interactions. It does require monitoring of blood pressure because dose-related hypertension may develop in some individuals. Venlafaxine prescribing in the United Kingdom has been restricted to psychiatrists. Venlafaxine appears to carry a greater risk of lethal arrhythmias in instances of overdose relative to the SSRIs, but less risk than with the TCAs. Desvenlafaxine, a newer form of the medication, is started at its target dose of 50 mg/day orally and does not require upward titration although higher doses have been well studied and some patients benefit from 100 mg/day. Duloxetine may also result in small increases in blood pressure. Common side effects include dry mouth, dizziness, and fatigue. Inhibitors of 1A2 and 2D6 may increase duloxetine levels with a risk of toxicity. Milnacipran, approved for the treatment of fibromyalgia, and levomilnacipran, approved for the treatment of major depression, carry many of the side effects common to other SNRIs including a mild tachycardia, hypertension, sexual side effects, mydriasis, urinary constriction, and occasional abnormal bleeding. Levomilnacipran is started at 20 mg/day orally then increased to 40 mg/day after 2–3 days. The target dose is 40–120 mg given once daily. Milnacipran is typically started at 12.5 mg/day orally, titrated to 12.5 mg twice daily after 2 days, and then to 25 mg twice daily after 7 days. The target dose is typically 100–200 mg/day given in in two divided doses. While not approved for the treatment of major depression, the evidence suggests that milnacipran, like levomilnacipran, is an effective antidepressant agent.
Nefazodone appears to lack the anticholinergic effects of the TCAs and the agitation sometimes induced by SSRIs. Nefazodone should not be given with terfenadine, astemizole, or cisapride, which are not commercially available in the United States. Because nefazodone inhibits the liver’s cytochrome P450 3A4 isoenzymes, concurrent use of these medications can lead to serious QT prolongation, ventricular tachycardia, or death. Through the same mechanism of enzyme inhibition, nefazodone can elevate cyclosporine levels sixfold to tenfold. Nefazodone carries an FDA warning given its association with liver failure in rare cases. Pretreatment and ongoing monitoring of liver biochemical enzymes are indicated.
Mirtazapine is thought to enhance central noradrenergic and serotonergic activity with minimal sexual side effects compared with the SSRIs. Its action as a potent antagonist of histaminergic receptors may make it a useful agent for patients with depression and insomnia. It is also an effective antiemetic due to its antagonism of the 5-HT3 receptor. Its most common adverse side effects include somnolence, increased appetite, weight gain, lipid abnormalities, and dizziness. The labeling for mirtazapine indicated that agranulocytosis was seen in 2 of 2796 patients in premarketing studies. An association of agranulocytosis or a clinically significant neutropenia with the medication appears to be modest. Although it is metabolized by P450 isoenzymes, it is not an inhibitor of this system. It is given in a single oral dose at bedtime starting at 15 mg and titrated up to 45 mg.
Vortioxetine is an antidepressant that blocks serotonin reuptake, is a partial agonist of the 5-HT1A receptor, and effects a variety of other serotonin receptor sites. The side effects attributed to its serotonergic effects include gastrointestinal upset and sexual dysfunction. Vortioxetine has demonstrated efficacy in improving cognitive symptoms of depression and received regulatory approval for this indication in Europe. Vortioxetine is typically dosed at 10 mg/day orally and may be increased to 20 mg/day.
2. Tricyclic antidepressants (TCAs) and clinically similar medications—TCAs were the mainstay of medication therapy for depression for many years. They have also been effective in panic disorder, pain syndromes, and anxiety states. Specific ones have been studied and found to be effective in OCD (clomipramine), enuresis (imipramine), psychotic depression (amoxapine), and reduction of craving in cocaine withdrawal (desipramine).
TCAs are characterized more by their similarities than by their differences. They tend to affect both serotonin and norepinephrine reuptake; some medications act mainly on the former and others principally on the latter neurotransmitter system. Individuals receiving the same dosages vary markedly in therapeutic drug levels achieved (elderly patients require smaller doses), and determination of plasma drug levels is helpful when clinical response has been disappointing. Nortriptyline is usually effective when plasma levels are between 50 and 150 ng/mL; imipramine at plasma levels of 200–250 ng/mL; and desipramine at plasma levels of 100–250 ng/mL. High blood levels are not more effective than moderate levels and may be counterproductive (eg, delirium, seizures). Patients with gastrointestinal side effects benefit from plasma level monitoring to assess absorption of the drug. Most TCAs can be given in a single dose at bedtime, starting at fairly low doses (eg, nortriptyline 25 mg orally) and increasing by 25 mg every several days as tolerated until the therapeutic response is achieved (eg, nortriptyline, 100–150 mg) or to maximum dose if necessary (eg, nortriptyline, 150 mg). The most common cause of treatment failure is an inadequate trial. A full trial consists of giving a therapeutic daily dosage for at least 6 weeks. Because of marked anticholinergic and sedating side effects, clomipramine is started at a low dose (25 mg/day orally) and increased slowly in divided doses up to 100 mg/day, held at that level for several days, and then gradually increased as necessary up to 250 mg/day. The TCAs have anticholinergic side effects to varying degrees (amitriptyline 100 mg is equivalent to atropine 5 mg). One must be particularly wary of the effect in elderly men with prostatic hyperplasia. The anticholinergic effects also predispose to other medical problems such as constipation, confusion, heat stroke, or dental problems from xerostomia. Orthostatic hypotension is fairly common, is not dose-dependent, and may not remit with time on medication; this may predispose to falls and hip fractures in the elderly.
Cardiac effects of the TCAs are functions of the anticholinergic effect, direct myocardial depression, quinidine-like effect, and interference with adrenergic neurons. These factors may produce altered rate, rhythm, and contractility, particularly in patients with preexisting cardiac disease, such as bundle-branch or bifascicular block. Even relatively small overdoses (eg, 1500 mg of imipramine) have resulted in lethal arrhythmias. Electrocardiographic changes range from benign ST segment and T wave changes and sinus tachycardia to a variety of complex and serious arrhythmias, the latter requiring a change in medication. Because TCAs have class I antiarrhythmic effects, they should be used with caution in patients with ischemic heart disease, arrhythmias, or conduction disturbances. SSRIs or the atypical antidepressants are better initial choices for this population.
TCAs lower the seizure threshold, so this is of particular concern in patients with a propensity for seizures. Loss of libido and erectile, ejaculatory, and orgasmic dysfunction are common and can compromise compliance. Trazodone rarely causes priapism (1 in 9000), but when it occurs, it requires treatment within 12 hours (epinephrine 1:1000 injected into the corpus cavernosum). Delirium, agitation, and mania are infrequent complications of the TCAs but can occur. Sudden discontinuation of some of these medications can produce “cholinergic rebound,” manifested by headaches and nausea with abdominal cramps. Overdoses of TCAs are often serious because of the narrow therapeutic index and quinidine-like effects (see Chapter 38).
3. Monoamine oxidase inhibitors—The MAO inhibitors are generally used as third-line medications for depression (after a failure of SSRIs, SNRIs, TCAs, or the atypical antidepressants) because of the dietary and other restrictions required (Table 25–8). They should be considered third-line medications for refractory panic disorder as well as depression; however, this hierarchy has become more flexible since MAO inhibitor skin patches (selegiline) have become available. They deliver the MAO inhibitor to the bloodstream bypassing the gastrointestinal tract so that dietary restrictions are not necessary in the lowest dosage strength (6 mg/24 h).
Table 25–8. Principal dietary restrictions in MAOI use.
1. Cheese, except cream cheese and cottage cheese and fresh yogurt
2. Fermented or aged meats such as bologna, salami
3. Broad bean pods such as Chinese bean pods
4. Liver of all types
5. Meat and yeast extracts
6. Red wine, sherry, vermouth, cognac, beer, ale
7. Soy sauce, shrimp paste, sauerkraut
MAOI, monoamine oxidase inhibitor.
The MAO inhibitors commonly cause symptoms of orthostatic hypotension (which may persist) and sympathomimetic effects of tachycardia, sweating, and tremor. Nausea, insomnia (often associated with intense afternoon drowsiness), and sexual dysfunction are common. Zolpidem 5–10 mg orally at bedtime can ameliorate MAO-induced insomnia. Central nervous system effects include agitation and toxic psychoses. Dietary limitations (see Table 25–8) and abstinence from medication products containing phenylpropanolamine, phenylephrine, meperidine, dextromethorphan, and pseudoephedrine are mandatory for MAO-A type inhibitors (those marketed for treatment of depression), since the reduction of available MAO leaves the patient vulnerable to exogenous amines (eg, tyramine in foodstuffs).
4. Other medications and those under investigation—Dextroamphetamine (5–30 mg/day orally) and methylphenidate (10–45 mg/day orally) may be effective for the short-term treatment of some depressive symptoms in medically ill and geriatric patients. The stimulants are notable for rapid onset of action (hours) and a paucity of side effects (tachycardia, agitation) in most patients. They are usually given in two divided doses early in the day (eg, 7 am and noon) so as to avoid interfering with sleep. These agents may also be useful as adjunctive agents in refractory depression. Intravenous infusion of the dissociative anesthetic ketamine has been shown to lead to a rapid improvement in depressive symptoms in 50–70% of patients with depression. The effects of a single treatment are short-lived (about 3–7 days). Ketamine acts both through the glutamate system as well as the opioid system. Other NMDA antagonists and opioid system agents continue to be evaluated in the treatment of resistant depression. Esketamine nasal spray has been approved by the FDA for the treatment of depression for patients who have been inadequately treated by two other antidepressant medications. However, there are ongoing concerns about long-term use of esketamine or ketamine, including the risk of abuse and longer-term impacts on mood and suicidality.
Allopregnanolone, a neurosteroid, is an allosteric modulator of GABA-a receptors and was approved in 2019 for the treatment of postpartum depression. Like ketamine, allopregnanolone is administered intravenously, and the antidepressant effects are rapid. An allopregnanolone infusion is given over 60 hours in a healthcare facility and was significantly more effective than placebo in treating postpartum depression by the end of the 2.5-day infusion, and benefits were sustained for the 30 days of the study period in three registration trials. The most common side effects of allopregnanolone are headache, dizziness, and somnolence. There is a rare side effect of loss of consciousness that requires the infusion be monitored in a healthcare facility. There is good reason to believe that GABA-a modulators will be useful in other types of depression, including for addressing anxiety and insomnia in patients with more severe depression. Several orally active GABA-a modulators are in development.
5. Switching and combination therapy—If the therapeutic response has been poor after an adequate trial with the chosen medication, the diagnosis should be reassessed. Assuming that the trial has been adequate and the diagnosis is correct, a trial with a second medication is appropriate. In switching from one group to another, an adequate “washout time” must be allowed. This is critical in certain situations—eg, in switching from a MAO inhibitor to a TCA, allow 2–3 weeks between stopping one medication and starting another; in switching from an SSRI to a MAO inhibitor, allow 4–5 weeks for fluoxetine and at least 2 weeks for other SSRIs. In switching within groups—eg, from one TCA to another (amitriptyline to desipramine, etc)—no washout time is needed, and one can rapidly decrease the dosage of one medication while increasing the other. In clinical practice, adjunctive treatment with lithium, buspirone, or thyroid hormone may be helpful in depression. The adjunctive use of low-dose atypical antipsychotics such as aripiprazole, olanzapine, and quetiapine in the treatment of patients with refractory depression is supported by research. The side effect risk is the same as when treating psychosis. Adding an atypical agent requires monitoring body mass index, lipids, and glucose. Combining two antidepressants, or adding an antipsychotic to an antidepressant, requires caution and is usually reserved for clinicians who feel comfortable managing this or after psychiatric consultation.
6. Maintenance and tapering—When clinical relief of symptoms is obtained, medication is continued for 12 months in the effective maintenance dosage, which is the dosage required in the acute stage. The full dosage should be continued indefinitely when the individual has a first episode before age 20 or after age 50, is over age 40 with two episodes, has at least one episode after age 50, or has had three episodes at any age. Major depression generally should be considered a chronic/intermittent disease with most patients having relapses in time and some patients never fully recovering from a depressive episode. If the medication is being tapered, it should be done gradually over several months, monitoring closely for relapse.
7. Drug interactions—Interactions with other medications are listed in Table 25–9.
Table 25–9. Antidepressant drug interactions with other medications (listed in alphabetical order within classes).
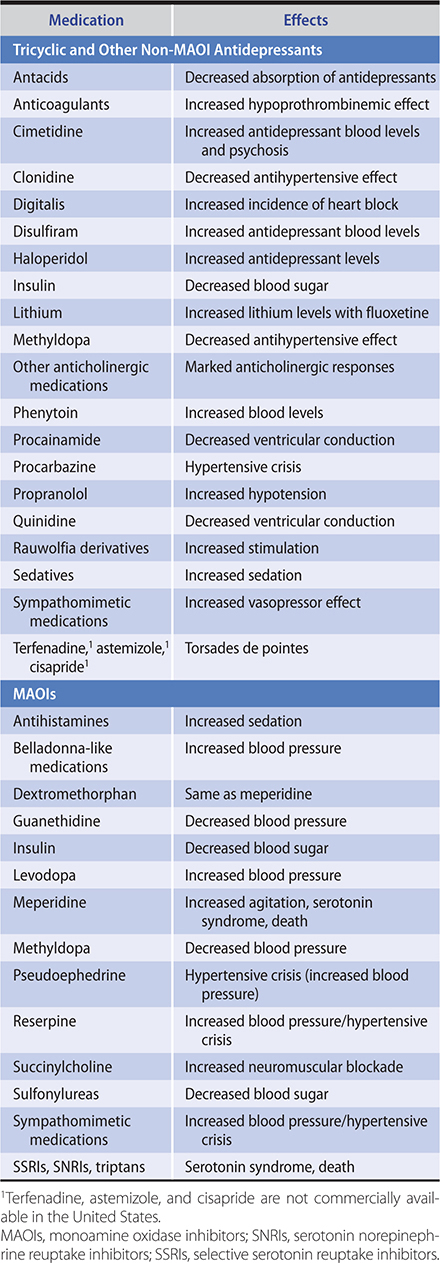
8. Electroconvulsive therapy—ECT causes a generalized central nervous system seizure (peripheral convulsion is not necessary) by means of electric current. The key objective is to exceed the seizure threshold, which can be accomplished by a variety of means. The mechanism of action is not known, but it is thought to involve major neurotransmitter responses at the cell membrane.
ECT is the most effective (about 45–85% remission rate) treatment of severe depression. In more treatment-resistant depression, remission rates from ECT are lower (around 48%). It is particularly effective for the delusions and agitation commonly seen with depression in the elderly. It is indicated when medical conditions preclude the use of antidepressants, nonresponsiveness to these medications, and extreme suicidality. Comparative controlled studies of ECT in severe depression show that it is more effective than pharmacotherapy. It is also effective in the treatment of mania and catatonia. It has also been shown to be helpful in chronic schizophrenic disorders when clozapine alone is not fully effective.
The most common side effects of ECT are memory disturbance and headache. Memory loss or confusion is usually related to the number and frequency of ECT treatments and proper oxygenation during treatment. Unilateral ECT is associated with less memory loss than bilateral ECT. Both anterograde and retrograde memory loss may occur, but short-term anterograde memory loss is more common. While some memory deficits may persist, memory loss tends to improve in a few weeks after the last ECT treatment.
Increased intracranial pressure is a contraindication. Other problems such as cardiac disorders, aortic aneurysms, bronchopulmonary disease, and venous thrombosis must be evaluated in light of the severity of the medical problem versus the need for ECT. Serious complications arising from ECT occur in less than 1 in 1000 cases. Most of these problems are cardiovascular or respiratory in nature (eg, aspiration of gastric contents, arrhythmias, myocardial infarction). Poor patient understanding and lack of acceptance of the technique by the public are some of the biggest obstacles to the use of ECT.
9. Phototherapy—Phototherapy is used in major depression with seasonal onset. It consists of indirect eye exposure to a light source of greater than 2500 lux for 2 hours daily or 10,000 lux for 20 minutes daily to increase the photoperiod of the day. Light visors are an adaptation that provides greater mobility and an adjustable light intensity but may not be as effective. The dosage varies, with some patients requiring morning and night exposure. One effect is alteration of biorhythm through melatonin mechanisms.
10. Repetitive transcranial magnetic stimulation—Repetitive transcranial magnetic stimulation (rTMS) is used to treat nonpsychotic treatment-resistant depression and involves the application of electromagnetic pulses to the dorsolateral prefrontal cortex. Its use in depression is approved by the FDA for individuals who have not tolerated or responded to at least one or more standard antidepressant medications. It is usually delivered in a course of 30 sessions over 6 weeks. rTMS neither requires general anesthesia nor is it associated with cognitive side effects. Several meta-analyses have demonstrated that in nonpsychotic depression, rTMS is noninferior to ECT. There is a small risk of seizure (1:30,000 in post market research), and this is due primarily to operator error. The most common side effects are scalp sensitivity under the coil and transient headache.
11. Other treatments—Vagus nerve stimulation has shown promise in about one-third of extremely refractory cases and is approved by the FDA but has not been approved by insurers. Data have demonstrated that the effects plateau around 18 months to 2 years and are durable at 5 years. Deep brain stimulation continues to be explored for the treatment of refractory depression but two multisite randomized controlled studies in two separate targets (subgenual cingulate and ventral capsule/ventral striatum) have not shown success to date. There has been one successful trial but the study methodology was a derivation of the traditional randomized controlled trial. This is still considered an experimental approach, and the appropriate target and methodology are unknown.
B. Psychological
It is often challenging to engage an individual in penetrating psychotherapeutic endeavors during the acute stage of a severe depression. While medications may be taking effect, a supportive and behavioral approach to strengthen existing coping mechanisms and appropriate consideration of the patient’s continuing need to function at work, to engage in recreational activities, etc, are necessary as the severity of the depression lessens. Therapy during or just after the acute stage may focus on coping techniques, with some practice of alternative choices. Depression-specific psychotherapies help improve self-esteem, increase assertiveness, and lessen dependency. Interpersonal psychotherapy for depression has shown efficacy in the treatment of acute depression, helping patients master interpersonal stresses and develop new coping strategies. Cognitive behavioral therapy for depression addresses patients’ patterns of negative thoughts, called cognitive distortions, which lead to feelings of depression and anxiety. Treatment usually includes homework assignments such as keeping a journal of cognitive distortions and of positive responses to them. The combination of medication therapy plus interpersonal psychotherapy or cognitive behavioral therapy is generally more effective than either modality alone. It is sometimes helpful to involve the spouse or other significant family members early in treatment. Mindfulness-based cognitive therapy has reduced relapse rates in several randomized controlled trials. In two studies, it was as effective as maintenance medication in preventing relapse. This therapy incorporates meditation and teaches patients to distance themselves from depressive thinking.
C. Social
Flexible use of appropriate social services can be of major importance in the treatment of depression. Since alcohol abuse is often associated with depression, early involvement in alcohol treatment programs such as Alcoholics Anonymous can be important to future success (see Alcohol Use Disorder [Alcoholism]). The structuring of daily activities during severe depression is often quite difficult for the patient, and loneliness is often a major factor. The help of family, employer, or friends is often necessary to mobilize the patient who experiences no joy in daily activities and tends to remain uninvolved and to deteriorate. Insistence on sharing activities will help involve the patient in simple but important daily functions. In some severe cases, the use of day treatment centers or support groups of a specific type (eg, mastectomy groups) is indicated. It is not unusual for a patient to have multiple legal, financial, and vocational problems requiring legal and vocational assistance.
D. Behavioral
When depression is a function of self-defeating coping techniques such as passivity, the role-playing approach can be useful. Behavioral techniques, including desensitization, may be used in problems such as phobias where depression is a by-product. When depression is a regularly used interpersonal style, behavioral counseling to family members or others can help in extinguishing the behavior in the patient. Behavioral activation, a technique of motivating depressed patients to begin engaging in pleasurable activities, has been shown to be a useful depression-specific psychotherapy. Exercise, especially aerobic and supervised by exercise professionals, has evidence in improving depressive symptoms.
 Treatment of Bipolar Disorder, Manic & Depressive Episodes
Treatment of Bipolar Disorder, Manic & Depressive Episodes
Acute manic or hypomanic symptoms will respond to the mood stabilizers lithium or valproic acid after several days of treatment. Antipsychotics may be used as well for mania. High-potency benzodiazepines (eg, clonazepam) may also be useful adjuncts in managing the agitation and sleep disturbance that are features of manic and hypomanic episodes.
A. Antipsychotics
Acute manic symptoms may be treated initially with a second-generation antipsychotic such as olanzapine, (eg, 5–20 mg orally), risperidone (2–3 mg orally), or aripiprazole (15–30 mg) in conjunction with a benzodiazepine if indicated. Alternatively, when behavioral control is immediately necessary, olanzapine in an injectable form (2.5–10 mg intramuscularly) or haloperidol, 5–10 mg orally or intramuscularly repeated as needed until symptoms subside, may be used. The dosage of the antipsychotic may be gradually reduced after lithium or another mood stabilizer is started. Olanzapine, quetiapine, ziprasidone, aripiprazole, and the long-acting injectable risperidone are approved as maintenance treatments for bipolar disorder to prevent subsequent cycles of both mania and depression.
B. Valproic Acid
Valproic acid (divalproex) is a first-line treatment for mania. This issue is particularly important in AIDS or other medically ill patients prone to dehydration or malabsorption with wide swings in serum lithium levels. Valproic acid has also been used effectively in panic disorder and migraine headache. Treatment is often started at a dose of 750 mg/day orally, and dosage is then titrated to achieve therapeutic serum levels. Oral loading in acutely manic bipolar patients in an inpatient setting (initiated at a dosage of 20 mg/kg/day) can safely achieve serum therapeutic levels in 2–3 days. Concomitant use of aspirin may increase valproate levels, carbamazepine or phenytoin may decrease valproate levels, while warfarin levels may be elevated by valproate. Gastrointestinal symptoms and weight gain are the main side effects. Liver enzyme biochemical tests, complete blood counts, glucose levels, and weight should be monitored at 2 weeks, 4 weeks, and 3 months initially and annually or more frequently thereafter based on clinical judgment. Significant teratogenic effects are a concern so pregnancy should be ruled out prior to initiation. In utero exposure to valproate has been associated with adversely affecting neural tube development in the fetus and there is an FDA warning to that effect. Thus, alternatives to valproate should be considered in women of childbearing years who might become pregnant.
C. Lithium
Lithium significantly decreases the frequency and severity of both manic and depressive attacks in about 50–70% of patients, and is FDA approved for maintenance and manic episodes.
In addition to its use in bipolar disorder, lithium is sometimes useful in the prophylaxis of recurrent unipolar depressions (perhaps undiagnosed bipolar disorder) and in lowering the risk of suicide. Lithium may ameliorate nonspecific aggressive behaviors and dyscontrol syndromes. Many patients with bipolar disease can be managed long-term with lithium alone, although some will require continued or intermittent use of an antipsychotic or lamotrigine to help prevent depressive episodes. An excellent resource for information is the Lithium Information Center, https://www.uwhealth.org/health/topic/multum/lithium/d00061a1.html.
Before treatment, the clinical workup should include a medical history and physical examination; complete blood count; T4, thyroid-stimulating hormone, blood urea nitrogen (BUN), serum creatinine, and serum electrolyte determinations; urinalysis; and electrocardiography (in patients over age 45 or with a history of cardiac disease).
1. Dosage—The common starting dosage of lithium carbonate is 300–900 mg daily, with trough blood levels measured after 4–5 days of treatment. A slow release form or units of different dosage may be used. Lithium citrate is available as a syrup. The dosage is that required to maintain blood levels in the therapeutic range. For acute manic episodes, this ranges from 0.8 mEq/L to 1.2 mEq/L. Although there is controversy about the optimal long-term maintenance dose, many clinicians reduce the acute level to 0.6–1 mEq/L in order to reduce side effects. The dose required to meet this need will vary in different individuals. Augmentation of antidepressants is usually achieved with lower blood levels. Once-a-day dosage is acceptable.
Lithium is readily absorbed, with peak serum levels occurring within 1–3 hours and complete absorption in 8 hours. Half of the total body lithium is excreted in 18–24 hours (95% in the urine). Blood for lithium levels should be drawn 12 hours after the last dose. Serum levels should be measured 4–7 days after initiation of treatment and changes in dose. For maintenance treatment, lithium levels should be monitored initially every 1–2 months but may be measured every 6–12 months in stable, long-term patients. Levels should be monitored more closely when there is any condition that causes volume depletion (eg, diarrhea, dehydration, use of diuretics).
2. Side effects—Early side effects, including mild gastrointestinal symptoms (take lithium with food and in divided doses), fine tremors (treat with propranolol, 20–60 mg/day orally, only if persistent), slight muscle weakness, and some degree of somnolence, can occur and are usually transient. Moderate polyuria (reduced renal responsiveness to ADH) and polydipsia (associated with increased plasma renin concentration) are often present. Potassium administration can blunt this effect, as may once-daily dosing of lithium. Weight gain (often a result of calories in fluids taken for polydipsia) and leukocytosis not due to infection can occur.
Thyroid side effects include goiter (3%; often euthyroid) and hypothyroidism (10%; concomitant administration of lithium and iodide or lithium and carbamazepine enhances the hypothyroid and goitrogenic effect of either medication). Most clinicians treat lithium-induced hypothyroidism (more common in women) with thyroid hormone while continuing lithium therapy. Changes in the glucose tolerance test toward a diabetes-like curve, nephrogenic diabetes insipidus (usually resolving about 8 weeks after cessation of lithium therapy), nephrotic syndrome, edema, folate deficiency, and pseudotumor cerebri (ophthalmoscopy is indicated if there are complaints of headache or blurred vision) can occur. Thyroid and kidney function should be checked at 4- to 6-month intervals. Hypercalcemia and elevated parathyroid hormone levels occur in some patients. Electrocardiographic abnormalities (principally T wave flattening or inversion) may occur during lithium administration but are not of major clinical significance. Sinoatrial block may occur, particularly in the elderly. Other medications that prolong intraventricular conduction, such as TCAs, must be used cautiously in conjunction with lithium. Lithium impairs ventilatory function in patients with airway obstruction. Lithium alone does not have a significant effect on sexual function, but when combined with benzodiazepines (clonazepam in most symptomatic patients), it causes sexual dysfunction in about 50% of men. Lithium may precipitate or exacerbate psoriasis in some patients and can also cause acne. Most of these side effects subside when lithium is discontinued; when residual side effects exist, they are usually not serious.
Side effects from long-term lithium therapy include the development of cogwheel rigidity and, occasionally, other extrapyramidal signs. Lithium potentiates the parkinsonian effects of haloperidol. Lithium-induced delirium with therapeutic lithium levels is an infrequent complication usually occurring in the elderly and may persist for several days after serum levels have become negligible. Encephalopathy has occurred in patients receiving combined lithium and antipsychotic therapy and in those who have cerebrovascular disease, thus requiring careful evaluation of patients who develop neurotoxic signs at subtoxic blood levels.
Some reports have suggested that the long-term use of lithium may have adverse effects on kidney function (with interstitial fibrosis, tubular atrophy, or nephrogenic diabetes insipidus). Persistent polyuria should require an investigation of the kidney’s ability to concentrate urine. A rise in serum creatinine levels is an indication for in-depth evaluation of kidney function and consideration of alternative treatments if the individual can tolerate a change. Incontinence has been reported in women, apparently related to changes in bladder cholinergic-adrenergic balance.
Prospective studies suggest that the overall risk imposed by lithium in pregnancy may be overemphasized. However, lithium exposure in early pregnancy does minimally increase the frequency of rare congenital anomalies, notably Ebstein and other major cardiovascular anomalies. For women who take psychotropic medications who become pregnant, the decision to make a change in medication is complex and requires informed consent regarding the relative risks to the patient and fetus. Indeed, the risk of untreated bipolar disorder carries its own risks for pregnancy. Mothers who take lithium should use formula to feed their newborn, since concentration in breast milk is one-third to half that in serum.
Frank toxicity usually occurs at blood lithium levels greater than 2 mEq/L. Because sodium and lithium are reabsorbed at the same loci in the proximal renal tubules, any sodium loss (diarrhea, use of diuretics, or excessive perspiration) increases lithium levels. Symptoms and signs include vomiting and diarrhea, the latter exacerbating the problem since more sodium is lost and more lithium is absorbed. Other symptoms and signs, some of which may not be reversible, include tremors, marked muscle weakness, confusion, dysarthria, vertigo, choreoathetosis, ataxia, hyperreflexia, rigidity, lack of coordination, myoclonus, seizures, opisthotonos, and coma. Toxicity is more severe in the elderly, who should be maintained on slightly lower serum levels. Lithium overdosage may be accidental or intentional or may occur because of poor monitoring. Significant overdoses of lithium are typically managed with hemodialysis since the medication is excreted completely by the kidneys.
See Chapter 38 for the treatment of patients with massive ingestions of lithium or blood lithium levels greater than 2.5 mEq/L.
3. Drug interactions—Patients receiving lithium should use diuretics with caution and only under close medical supervision. The thiazide diuretics cause increased lithium reabsorption from the proximal renal tubules, resulting in increased serum lithium levels (Table 25–10), and adjustment of lithium intake must be made to compensate for this. Reduce lithium dosage by 25–40% when the patient is receiving 50 mg of hydrochlorothiazide daily. Potassium-sparing diuretics (spironolactone, amiloride, triamterene) may also increase serum lithium levels and require careful monitoring of lithium levels. Loop diuretics (furosemide, ethacrynic acid, bumetanide) do not appear to alter serum lithium levels. Concurrent use of lithium and angiotensin-converting enzyme inhibitors requires a 50–75% reduction in lithium intake, as does prolonged concurrent use of nonsteroidal anti-inflammatory medication.
Table 25–10. Lithium interactions with other medications (listed in alphabetical order).
D. Carbamazepine
Carbamazepine is used in the treatment of bipolar patients who cannot be satisfactorily treated with lithium (nonresponsive, excessive side effects, or rapid cycling). It is often effective at 800–1600 mg/day orally. It has also been used in the treatment of trigeminal neuralgias and alcohol withdrawal as well as in patients with behavioral dyscontrol. It has been used to treat residual symptoms in previous stimulant abusers (eg, PTSD with impulse control problems). Dose-related side effects include sedation and ataxia. Dosages start at 400–600 mg orally daily and are increased slowly to therapeutic levels. Skin rashes and a mild reduction in white count are common. SIADH occurs rarely. Nonsteroidal anti-inflammatory medications (except aspirin), the antibiotics erythromycin and isoniazid, the calcium channel blockers verapamil and diltiazem (but not nifedipine), fluoxetine, propoxyphene, and cimetidine all increase carbamazepine levels. Carbamazepine can be effective in conjunction with lithium, although there have been reports of reversible neurotoxicity with the combination. Carbamazepine stimulates hepatic microsomal enzymes and so tends to decrease levels of haloperidol and oral contraceptives. It also lowers T4, free T4, and T3 levels. Cases of fetal malformation (particularly spina bifida) have been reported along with growth deficiency and developmental delay. Liver biochemical tests and complete blood counts should be monitored in patients taking carbamazepine. Genetic studies suggest that screening for the HLA-B1502 allele in the Han Chinese population and the HLA-A3101 allele in northern Europeans may help target individuals more susceptible to a serious rash. Oxcarbazepine, a derivative of carbamazepine, does not appear to induce its own metabolism, and is associated with fewer drug interactions, although it may impose a higher risk of hyponatremia. FDA-approved for partial seizures, oxcarbazepine may have efficacy in acute mania. It appears to be a safer alternative to carbamazepine due to its lower risk of hepatotoxicity.
E. Lamotrigine
Lamotrigine is thought to inhibit neuronal sodium channels and the release of the excitatory amino acids, glutamate and aspartate. It is FDA-approved for the maintenance treatment of bipolar disorder. Two double-blind studies support its efficacy in the treatment of acute bipolar depression as adjunctive therapy or as monotherapy, but several other controlled studies failed to demonstrate benefit. Likewise, lamotrigine has not proven effective in the management of acute mania. Its metabolism is inhibited by coadministration of valproic acid—doubling its half-life—and accelerated by hepatic enzyme-inducing agents such as carbamazepine. More frequent mild side effects include headache, dizziness, nausea, and diplopia. Rash occurring in 10% of patients may be an indication for immediate cessation of dosing, since lamotrigine has been associated with Stevens-Johnson syndrome (1:1000) and, rarely, toxic epidermal necrolysis. The medication should be stopped for a rash associated with systemic symptoms including fever, lymphadenopathy, and oral mucosa ulcerations, and the patient sent to an emergency department. Any new rash associated with lamotrigine use should be evaluated by a dermatologist. Dosing starts at 25–50 mg/day orally and is titrated upward slowly to decrease the likelihood of rash. Slower titration and a lower total dose are indicated for patients taking valproic acid.
 Prognosis
Prognosis
Most depressive episodes are usually time-limited, and the prognosis with treatment is good if a pathologic pattern of adjustment does not intervene. Major affective disorders frequently respond well to a full trial of medication treatment. However, at least 20% of patients will have a more chronic illness lasting 2 or more years. Many patients do not sustain a complete remission of symptoms and most depressive episodes recur. At least 80% of patients who have a single major depressive episode will have one or more recurrences within 15 years of the index episode. Many patients, therefore, require long-term maintenance therapy with antidepressants.
Mania has a good prognosis with adequate treatment, although patient adherence to treatment is often quite challenging. Few effective medications exist for bipolar depression, which include quetiapine, lurasidone, cariprazine, and the combination of fluoxetine and olanzapine. Many patients with bipolar disorder require treatment with two or more medications such as lithium, antipsychotics, and sleeping agents. Breakthrough manic or depressive episodes are common, even with adherence to maintenance treatments, although maintenance therapy lessens the risk of recurrent episodes.
Cipriani A et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018 Apr 7;391(10128):1357–66. [PMID: 29477251]
Daly EJ et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2018 Feb 1;75(2):139–48. [PMID: 29282469]
Fountoulakis KN et al. The International College of Neuro-Psychopharmacology (CINP) treatment guidelines for bipolar disorder in adults (CINP-BD-2017), part 3: the clinical guidelines. Int J Neuropsychopharmacol. 2017 Feb 1;20(2):180–95. [PMID: 27941079]
Lähteenvuo M et al. Real-world effectiveness of pharmacologic treatments for the prevention of rehospitalization in a Finnish nationwide cohort of patients with bipolar disorder. JAMA Psychiatry. 2018 Apr 1;75(4):347–55. [PMID: 29490359]
Leader LD et al. Brexanolone for postpartum depression: clinical evidence and practical considerations. Pharmacotherapy. 2019 Nov;39(11):1105–12. [PMID: 31514247]
Meltzer-Brody S et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018 Sep 22;392(10152):1058–70. Erratum in: Lancet. 2018 Sep 29;392(10153):1116. [PMID: 30177236]
Molero P et al. Antidepressant efficacy and tolerability of ketamine and esketamine: a critical review. CNS Drugs. 2018 May;32(5):411–20. [PMID: 29736744]
Viktorin A et al. Association of antidepressant medication use during pregnancy with intellectual disability in offspring. JAMA Psychiatry. 2017 Oct 1;74(10):1031–8. [PMID: 28700807]
Williams NR et al. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry. 2018 Dec 1;175(12):1205–15. [PMID: 30153752]
ATTENTION-DEFICIT/HYPERACTIVITY DISORDER
ESSENTIALS OF DIAGNOSIS

 Persistent patterns of inability to sustain attention, excessive motor activity/restlessness/impulsivity, or both.
Persistent patterns of inability to sustain attention, excessive motor activity/restlessness/impulsivity, or both.
 Symptoms interfere with daily functioning.
Symptoms interfere with daily functioning.
 Symptoms began prior to age 12 and in at least two settings (ie, school/work, home, with friends/family).
Symptoms began prior to age 12 and in at least two settings (ie, school/work, home, with friends/family).
 Clinical Findings
Clinical Findings
While attention-deficit/hyperactivity disorder (ADHD) begins in childhood, symptoms persist into adulthood in approximately two-thirds of patients, with half of those still requiring medication to aid in their functioning. The prevalence of ADHD in adults is estimated to be 4–5%. ADHD is never diagnosed in some patients during childhood because they may not have presented for assessment at that time or were able to compensate for symptoms at the time. The specific presenting symptoms in adulthood tend to be inattention, restlessness, and impulsivity, whereas hyperactivity has often improved. At least five inattention symptoms (such as making careless mistakes, being easily sidetracked, trouble keeping deadlines or with organization, losing belongings, being forgetful in daily chores/tasks) are required to meet criteria for this subtype of ADHD, or five hyperactivity/impulsivity symptoms (such as feeling restless and leaving a seat though expected to remain, feeling “driven by a motor,” interrupting others, cannot wait his or her turn) for this subtype. It is often useful to have patients provide questionnaires to other adult observers, including those who knew them during childhood, such as parents. This collateral data can help prevent diagnosing ADHD in someone who is seeking stimulants but without symptomatology as well as aid in making the diagnosis, since evidence shows that many adults who do have ADHD often underreport symptoms.
 Treatment
Treatment
A. Pharmacologic
Stimulants such as methylphenidate and amphetamine are the most effective treatment, with some of the largest effect sizes for medication treatment in psychiatric disorders. These come in both short-acting and long-acting formulations. Caution should be used to assess for potential substance abuse or diversion as well as for comorbid mood disorders that may not respond well to a stimulant prior to prescribing these medications. Atomoxetine, a nonstimulant, is a second-line agent that is FDA-approved for ADHD; it affects norepinephrine and dopamine transport and makes more of these neurotransmitters available in the brain. Bupropion has evidence of efficacy as well and may be considered in patients in whom a stimulant is contraindicated or in those who also suffer from major depression. Desipramine, a tricyclic antidepressant, also can be effective for ADHD and may be considered in patients who have additional needs, such as a concomitant depression or neuropathic pain. Guanfacine and clonidine are two additional nonstimulant medications used primarily to treat blood pressure but with some efficacy in ADHD as well.
B. Behavioral and Other Treatments
Psychoeducation regarding ADHD should be given to all patients. Many patients are able to implement behavioral changes that either improve their functioning, such as creating calendars and organizational schemes or doing tasks in multiple timed short spurts, or can help them avoid tasks that are challenging for them in favor of complementary tasks they are more suited to (ie, selecting jobs that value more activity rather than sustained focus, or sharing in the chores at home that do not require attention to detail). Cognitive behavioral therapy has some evidence for helping residual symptoms after medication management has been optimized.
Cortese S et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. Lancet Psychiatry. 2018 Sep;5(9):727–38. [PMID: 30097390]
AUTISM SPECTRUM DISORDERS
ESSENTIALS OF DIAGNOSIS

 Persistent issues with social communication and interactions.
Persistent issues with social communication and interactions.
 Repetitive behaviors, interests, or activities.
Repetitive behaviors, interests, or activities.
 Symptoms interfere with functioning.
Symptoms interfere with functioning.
 May or may not have accompanying language or intellectual impairment.
May or may not have accompanying language or intellectual impairment.
 Clinical Findings
Clinical Findings
Autism spectrum disorder is a neurodevelopmental disorder in which patients suffer from pervasive difficulties with social communication and have repetitive, restricted interests and behaviors. Autism spectrum disorder affects about 1% of the adult population with an estimated heritability of about 90%. Approximately 20–30% of individuals in whom autism is diagnosed also have a substance use problem as well as a higher risk of ADHD, mood, or obsessive-compulsive disorders. The National Institute of Health and Care Excellence (NICE) guidelines recommend that assessment of autism spectrum disorder should be a comprehensive and multidisciplinary approach that includes asking about core autism spectrum disorder difficulties, early development, medical and family history, behavior, education, employment, needs assessment, risks, physical examination with potential laboratory testing, and feedback to the individual.
 Treatment
Treatment
No treatments for the core symptoms of autism spectrum disorder in adults have been validated. Two antipsychotics, risperidone and paliperidone, are approved for treating irritability in patients with autism spectrum disorders. These antipsychotics can help with some of the behavioral symptoms of autism but also carry a risk of metabolic side effects and extrapyramidal symptoms. There is some evidence for therapy, such as applied behavioral analysis, to address social cognitions and behaviors. Use of repetitive transcranial magnetic stimulation and vasopressin for the treatment of autism spectrum disorder are under investigation.
Howes OD et al. Autism spectrum disorder: consensus guidelines on assessment, treatment and research from the British Association for Psychopharmacology. J Psychopharmacol. 2018 Jan;32(1):3–29. [PMID: 29237331]
Umbricht D et al. A single dose, randomized, controlled proof-of-mechanism study of a novel vasopressin 1a receptor antagonist (RG7713) in high-functioning adults with autism spectrum disorder. Neuropsychopharmacology. 2017 Aug;42(9):1914–23. [PMID: 27711048]
SLEEP-WAKE DISORDERS
Sleep consists of two distinct states as shown by electroencephalographic studies: (1) REM (rapid eye movement) sleep, also called dream sleep, D state sleep, or paradoxic sleep, and (2) NREM (non-REM) sleep, also called S stage sleep, which is divided into stages 1, 2, 3, and 4 and is recognizable by different electroencephalographic patterns. Stages 3 and 4 are “delta” sleep. Dreaming occurs mostly in REM and to a lesser extent in NREM sleep.
Sleep is a cyclic phenomenon, with four or five REM periods during the night accounting for about one-fourth of the total night’s sleep (1.5–2 hours). The first REM period occurs about 80–120 minutes after onset of sleep and lasts about 10 minutes. Later REM periods are longer (15–40 minutes) and occur mostly in the last several hours of sleep. Most stage 4 (deepest) sleep occurs in the first several hours.
Age-related changes in normal sleep include an unchanging percentage of REM sleep and a marked decrease in stage 3 and stage 4 sleep, with an increase in wakeful periods during the night. These normal changes, early bedtimes, and daytime naps play a role in the increased complaints of insomnia in older people. Variations in sleep patterns may be due to circumstances (eg, “jet lag”) or to idiosyncratic patterns (“night owls”) in persons who perhaps because of different “biologic rhythms” habitually go to bed late and sleep late in the morning. Creativity and rapidity of response to unfamiliar situations are impaired by loss of sleep. There are also rare individuals who have chronic difficulty in adapting to a 24-hour sleep-wake cycle (desynchronization sleep disorder), which can be resynchronized by altering exposure to light.
The three major sleep disorders are discussed below. Any persistent sleep disorder that is not attributable to another condition should be evaluated by a sleep specialist.
1. Insomnia
 Classification & Clinical Findings
Classification & Clinical Findings
Patients may complain of difficulty getting to sleep or staying asleep, intermittent wakefulness during the night, early morning awakening, or combinations of any of these. Transient episodes are usually of little significance. Stress, caffeine, physical discomfort, daytime napping, and early bedtimes are common factors.
Psychiatric disorders are often associated with persistent insomnia. Depression is usually associated with fragmented sleep, decreased total sleep time, earlier onset of REM sleep, a shift of REM activity to the first half of the night, and a loss of slow wave sleep—all of which are nonspecific findings. In manic disorders, a reduced total sleep time and a decreased need for sleep are cardinal features and important early sign of impending mania. In addition to a decreased amount of sleep, manic episodes are characterized by a shortened REM latency and increased REM activity. Sleep-related panic attacks occur in the transition from stage 2 to stage 3 sleep in some patients with a longer REM latency in the sleep pattern preceding the attacks.
Abuse of alcohol may cause or be secondary to the sleep disturbance. There is a tendency to use alcohol as a means of getting to sleep without realizing that it disrupts the normal sleep cycle. Acute alcohol intake produces a decreased sleep latency with reduced REM sleep during the first half of the night. REM sleep is increased in the second half of the night, with an increase in total amount of slow wave sleep (stages 3 and 4). Vivid dreams and frequent awakenings are common. Chronic alcohol abuse increases stage 1 and decreases REM sleep (most medications delay or block REM sleep), with symptoms persisting for many months after the individual has stopped drinking. Acute alcohol or other sedative withdrawal causes delayed onset of sleep and REM rebound with intermittent awakening during the night.
Heavy smoking (more than a pack a day) causes difficulty falling asleep—apparently independently of the often associated increase in coffee drinking. Excess intake near bedtime of caffeine, cocaine, and other stimulants (eg, over-the-counter cold remedies) causes decreased total sleep time—mostly NREM sleep—with some increased sleep latency.
Sedative-hypnotics—specifically, the benzodiazepines, which are the most commonly prescribed medications to promote sleep—tend to increase total sleep time, decrease sleep latency, and decrease nocturnal awakening, with variable effects on NREM sleep. Nonbenzodiazepine hypnotics have similar effects on sleep as do the benzodiazepines, though some evidence shows improved slow wave sleep and less residual next-morning somnolence with non-benzodiazepines, such as zolpidem. Withdrawal causes just the opposite effects and results in continued use of the drug for the purpose of preventing withdrawal symptoms. Antidepressants decrease REM sleep (with marked rebound on withdrawal in the form of nightmares) and have varying effects on NREM sleep. The effect on REM sleep correlates with reports that REM sleep deprivation produces improvement in some depressions.
Persistent insomnias are also related to a wide variety of medical conditions, particularly delirium, pain, respiratory distress syndromes, uremia, asthma, thyroid disorders, and nocturia due to benign prostatic hyperplasia. Sleep apnea and restless leg movement are described below. Adequate analgesia and proper treatment of medical disorders will reduce symptoms and decrease the need for sedatives.
 Treatment
Treatment
In general, there are two broad classes of treatment for insomnia, and the two may be combined: psychological (cognitive-behavioral) and pharmacologic. In situations of acute distress, such as a grief reaction, pharmacologic measures may be most appropriate. With primary insomnia, however, initial efforts should be psychologically based. This is particularly true in the elderly to avoid the potential adverse reactions of medications. The elderly population is at risk for complaints of insomnia because sleep becomes lighter and more easily disrupted with aging. Medical disorders that become more common with age may also predispose to insomnia.
A. Psychological
Psychological strategies should include educating the patient regarding good sleep hygiene: (1) Go to bed only when sleepy. (2) Use the bed and bedroom only for sleeping and sex. (3) If still awake after 20 minutes, leave the bedroom, pursue a restful activity (such as a bath or meditation), and only return when sleepy. (4) Get up at the same time every morning regardless of the amount of sleep during the night. (5) Discontinue caffeine and nicotine, at least in the evening if not completely. (6) Establish a daily exercise regimen. (7) Avoid alcohol as it may disrupt continuity of sleep. (8) Limit fluids in the evening. (9) Learn and practice relaxation techniques. (10) Establish a bedtime ritual and a routine time for going to sleep. Research suggests that cognitive behavioral therapy for insomnia is as effective as zolpidem with benefits sustained 1 year after treatment.
B. Pharmacologic
When the above measures are insufficient, medications may be useful. Lorazepam (0.5 mg orally nightly), temazepam (7.5–15 mg orally nightly), and the nonbenzodiazepine hypnotics zolpidem (5–10 mg orally nightly, with a limit of 5 mg indicated for women) and zaleplon (5–10 mg orally nightly) are often effective for the elderly population and can be given in larger doses—twice what is prescribed for the elderly—in younger patients. Zaleplon is often used to treat insomnia characterized by middle-of-the-night awakening with difficulty falling back to sleep. Eszopiclone (2–3 mg orally) is similar in action to zolpidem and zaleplon and has a longer duration of action. A lower dose of 1 mg is indicated in the elderly or those with hepatic impairment. It is important to note that short-acting agents like triazolam or zolpidem may lead to amnestic episodes if used on a daily ongoing basis. Longer-acting agents such as flurazepam (half-life of more than 48 hours) may accumulate in the elderly and lead to cognitive slowing, ataxia, falls, and somnolence. In general, it is appropriate to use medications for short courses of 1–2 weeks. The medications described above have largely replaced barbiturates as hypnotic agents because of their greater safety in overdose and their lesser hepatic enzyme induction effects. Antihistamines such as diphenhydramine (25 mg orally nightly) or hydroxyzine (25 mg orally nightly) may also be useful for sleep, as they produce no pharmacologic dependency; their anticholinergic effects may, however, produce confusion or urinary symptoms in the elderly. Trazodone, an atypical antidepressant, is a non–habit-forming, effective sleep medication in lower than antidepressant doses (25–150 mg orally at bedtime). Priapism is a rare side effect requiring emergent treatment. Doxepin, 3–6 mg per night, is a TCA that is also effective for insomnia. Ramelteon, 8 mg orally at bedtime, is a melatonin receptor agonist that helps with sleep onset and does not appear to have abuse potential. It appears to be safe for ongoing use without the development of tolerance.
The dual orexin receptor antagonists (DORAs) class of hypnotics are approved to help initiate and maintain sleep. DORAs such as suvorexant may be more effective than other hypnotics for some patients. However, the role of suvorexant has not been established relative to other hypnotics and is more expensive since it is not generically available. DORAs have shown a significant increase in depressive symptoms in a subset of patients, so other hypnotics may be a better choice in depressed patients. The dose of suvorexant is 10–20 mg orally given about 30 minutes before bedtime.
2. Hypersomnias (Disorders of Excessive Sleepiness)
 Classification & Clinical Findings
Classification & Clinical Findings
A. Breathing-Related Sleep Disorders
Obstructive sleep apnea is by far the most common of the breathing-related sleep disorders that include central sleep apnea and sleep-related hypoventilation. Obstructive sleep apnea hypopnea is characterized by snoring, gasping, or breathing pauses during sleep and five or more apneas or hypopneas per hour or evidence by polysomnography. (See Chapter 9.)
B. Narcolepsy Hypocretin Deficiency Syndrome
Narcolepsy consists of a tetrad of symptoms: (1) sudden, brief (about 15 minutes) sleep attacks that may occur during any type of activity; (2) cataplexy—sudden loss of muscle tone involving specific small muscle groups or generalized muscle weakness that may cause the person to slump to the floor, unable to move, often associated with emotional reactions and sometimes confused with seizure disorder; (3) sleep paralysis—a generalized flaccidity of muscles with full consciousness in the transition zone between sleep and waking; and (4) hypnagogic hallucinations, visual or auditory, which may precede sleep or occur during the sleep attack. The attacks are characterized by an abrupt transition into REM sleep—a necessary criterion for diagnosis. The disorder begins in early adult life, affects both sexes equally, and usually levels off in severity at about 30 years of age.
REM sleep behavior disorder, characterized by motor dyscontrol and often violent dreams during REM sleep, may be related to narcolepsy.
C. Kleine-Levin Syndrome
This syndrome, which occurs mostly in young men, is characterized by hypersomnic attacks three or four times a year lasting up to 2 days, with hyperphagia, hypersexuality, irritability, and confusion on awakening. It has often been associated with antecedent neurologic insults. It usually remits after age 40.
D. Periodic Limb Movement Disorder
Periodic lower leg movements occur only during sleep with subsequent daytime sleepiness, anxiety, depression, and cognitive impairment. Restless leg syndrome includes movements while awake as well.
E. Shift Work Sleep Disorder
Shift work sleep disorder occurs when there is excessive fatigue as a consequence of work occurring during the normal sleep period.
 Treatment
Treatment
Narcolepsy can be managed by daily administration of a stimulant such as dextroamphetamine sulfate, 10 mg orally in the morning, with increased dosage as necessary. Modafinil and its enantiomer armodafinil are schedule IV medications FDA-approved for treating the excessive daytime fatigue of narcolepsy, sleepiness associated with obstructive sleep apnea, as well as for shift work sleep disorder. Usual dosing is 200–400 mg orally each morning for modafinil and 150–250 mg orally in the morning for armodafinil. The exact mechanism of action of modafinil and armodafil is unknown, yet they are thought to be less of an abuse risk than stimulants that are primarily dopaminergic. Common side effects include headache and anxiety; however, modafinil appears to be generally well tolerated. Modafinil may reduce the efficacy of cyclosporine, oral contraceptives, and other medications by inducing their hepatic metabolism. Imipramine, 75–100 mg orally daily, has been effective in treatment of cataplexy but not narcolepsy.
Periodic limb movement disorder and REM sleep behavior disorder can be treated with clonazepam with variable results. There is no treatment for Kleine-Levin syndrome, although lithium can prevent recurrences in some.
Treatment of sleep apnea is discussed in Chapter 9.
3. Parasomnias (Abnormal Behaviors during Sleep)
These disorders (sleep terror, nightmares, sleepwalking, and enuresis) are fairly common in children but less so in adults.
Espie CA et al. Effect of digital cognitive behavioral therapy for insomnia on health, psychological well-being, and sleep-related quality of life: a randomized clinical trial. JAMA Psychiatry. 2019 Jan 1;76(1):21–30. [PMID: 30264137]
Krystal AD et al. The assessment and management of insomnia: an update. World Psychiatry. 2019 Oct;18(3):337–52. [PMID: 31496087]
Sateia MJ et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017 Feb 15;13(2):307–49. [PMID: 27998379]
DISORDERS OF AGGRESSION
Aggression and violence are symptoms rather than diseases, and most frequently they are not necessarily associated with an underlying medical condition. Clinicians are unable to predict dangerous behavior with greater than chance accuracy. Depression, schizophrenia, personality disorders, mania, paranoia, temporal lobe dysfunction, and organic mental states may be associated with acts of aggression. Impulse control disorders are characterized by physical abuse (usually of the aggressor’s domestic partner or children), by pathologic intoxication, by impulsive sexual activities, and by reckless driving. Anabolic steroid usage by athletes has been associated with increased tendencies toward violent behavior.
In the United States, a significant proportion of all violent deaths are alcohol related. The ingestion of even small amounts of alcohol can result in pathologic intoxication that resembles an acute organic mental condition. Amphetamines, crack cocaine, and other stimulants are frequently associated with aggressive behavior. Phencyclidine is a drug commonly associated with violent behavior that is occasionally of a bizarre nature, partly due to lowering of the pain threshold. Domestic violence and rape are much more widespread than previously recognized. Awareness of the problem is to some degree due to increasing recognition of the rights of women and the understanding by women that they do not have to accept abuse. Acceptance of this kind of aggressive behavior inevitably leads to more, with the ultimate aggression being murder—20–50% of murders in the United States occur within the family. Police are called more for domestic disputes than all other criminal incidents combined. Children living in such family situations frequently become victims of abuse.
Features of individuals who have been subjected to long-term physical or sexual abuse are as follows: trouble expressing anger, staying angry longer, general passivity in relationships, feeling “marked for life” with an accompanying feeling of deserving to be victimized, lack of trust, and dissociation of affect from experiences. They are prone to express their psychological distress with somatization symptoms, often pain complaints. They may also have symptoms related to posttraumatic stress, as discussed above. The clinician should be suspicious about the origin of any injuries not fully explained, particularly if such incidents recur.
 Treatment
Treatment
A. Psychological
Management of any acutely potentially violent individual includes appropriate psychological maneuvers. Move slowly, talk slowly with clarity and reassurance, and evaluate the situation. Strive to create a setting that is minimally disturbing, and eliminate people or things threatening to the violent individual. Do not threaten and do not touch or crowd the person. Allow no weapons in the area (an increasing problem in hospital emergency departments). Proximity to a door is comforting to both the patient and the examiner. Use a negotiator who the violent person can relate to comfortably. Food and drink are helpful in defusing the situation (as are cigarettes for those who smoke). Honesty is important. Make no false promises, bolster the patient’s self-esteem, and continue to engage the subject verbally until the situation is under control. This type of individual does better with strong external controls to replace the lack of inner controls over the long term. Close probationary supervision and judicially mandated restrictions can be most helpful. There should be a major effort to help the individual avoid drug use (eg, Alcoholics Anonymous). Victims of abuse are essentially treated as any victim of trauma and, not infrequently, have evidence of PTSD.
B. Pharmacologic
Pharmacologic means are often necessary whether or not psychological approaches have been successful. This is particularly true in the agitated or psychotic patient. The medications of choice in seriously violent or psychotic aggressive states are antipsychotics, given intramuscularly if necessary, every 1–2 hours until symptoms are alleviated. A number of second-generation intramuscular antipsychotics are FDA approved in the management of acute agitation, and include aripiprazole (9.75 mg/1.3 mL), ziprasidone (10 mg/0.5 mL), and olanzapine (10 mg/2 mL). The second-generation antipsychotics appear less likely than first-generation medications like haloperidol (2.5–5 mg) to cause acute extrapyramidal symptoms. However, the second-generation medications appear no more effective than first-generation medications and generally are more expensive. Benzodiazepine sedatives (eg, diazepam, 5 mg orally or intravenously every several hours) can be used for mild to moderate agitation, but are sometimes associated with a disinhibition of aggressive impulses similar to alcohol. Chronic aggressive states, particularly in intellectual disabilities and brain damage (rule out causative organic conditions and medications such as anticholinergic medications in amounts sufficient to cause confusion), have been ameliorated with risperidone, 0.5–2 mg/day orally, propranolol, 40–240 mg/day orally, or pindolol, 5 mg twice daily orally (pindolol causes less bradycardia and hypotension than propranolol). Carbamazepine and valproic acid are effective in the treatment of aggression and explosive disorders, particularly when associated with known or suspected brain lesions. Lithium and SSRIs are also effective for some intermittent explosive outbursts. Buspirone (10–45 mg/day orally) is helpful for aggression, particularly in patients with intellectual disabilities.
C. Physical
Physical management is necessary if psychological and pharmacologic means are not sufficient. It requires the active and visible presence of an adequate number of personnel (five or six) to reinforce the idea that the situation is under control despite the patient’s lack of inner controls. Such an approach often precludes the need for actual physical restraint. Seclusion rooms and restraints should be used only when necessary (ambulatory restraints are an alternative), and the patient must then be observed at frequent intervals. Narrow corridors, small spaces, and crowded areas exacerbate the potential for violence in an anxious patient.
D. Other Interventions
The treatment of victims (eg, battered women) is challenging and can be complicated by a reluctance to leave the situation. Reasons for staying vary, but common themes include the fear of more violence because of leaving, the hope that the situation may ameliorate (in spite of steady worsening), and the financial aspects of the situation, which are seldom to the woman’s advantage. Concerns for the children often finally compel the woman to seek help. An early step is to get the woman into a therapeutic situation that provides the support of others in similar straits. Al-Anon is frequently a valuable asset when alcohol is a factor. The group can support the victim while she gathers strength to consider alternatives without being paralyzed by fear. Many cities offer temporary emergency centers and counseling. Use the available resources, attend to any medical or psychiatric problems, and maintain a compassionate interest. Some states require clinicians to report injuries caused by abuse or suspected abuse to police authorities. See Chapter e6 for detailed discussion.
Lee AH et al. Anger and aggression treatments: a review of meta-analyses. Curr Opin Psychol. 2018 Feb;19:65–74. [PMID: 29279226]
Nash RP et al. Acute pharmacological management of behavioral and emotional dysregulation following a traumatic brain injury: a systematic review of the literature. Psychosomatics. 2019 Mar–Apr;60(2):139–52. [PMID: 30665668]
SUBSTANCE USE DISORDERS
The term “dependency” was previously used to describe a severe form of substance abuse and drug addiction characterized by the triad of: (1) a psychological dependence or craving and the behavior involved in procurement of the drug; (2) physiologic dependence, with withdrawal symptoms on discontinuance of the drug; and (3) tolerance, ie, the need to increase the dose to obtain the desired effects. The terms “dependency” and “abuse” were dropped in DSM-5 in favor of the single term “substance use disorder,” ranging from mild to severe. Many patients could have a severe and life-threatening abuse problem without ever being dependent on a drug.
There is accumulating evidence that an impairment syndrome exists in many former (and current) drug users. It is believed that drug use produces damaged neurotransmitter receptor sites and that the consequent imbalance produces symptoms that may mimic other psychiatric illnesses. “Kindling”—repeated stimulation of the brain—renders the individual more susceptible to focal brain activity with minimal stimulation. Stimulants and depressants can produce kindling, leading to relatively spontaneous effects no longer dependent on the original stimulus. These effects may be manifested as mood swings, panic, psychosis, and occasionally overt seizure activity. The imbalance also results in frequent job changes, partner problems, and generally erratic behavior. Patients with PTSD frequently have treated themselves with a variety of drugs. Chronic abusers of a wide variety of drugs exhibit cerebral atrophy on CT scans, a finding that may relate to the above symptoms. Early recognition is important, mainly to establish realistic treatment programs that are chiefly symptom-directed.
The clinician faces three problems with substance use disorders: (1) the prescribing of substances such as sedatives, stimulants, or opioids that might produce dependency; (2) the treatment of individuals who have already abused drugs, most commonly alcohol; and (3) the detection of illicit drug use in patients presenting with psychiatric symptoms. The usefulness of urinalysis for detection of drugs varies markedly with different drugs and under different circumstances (pharmacokinetics is a major factor). Water-soluble drugs (eg, alcohol, stimulants, opioids) are eliminated in a day or so. Lipophilic substances (eg, barbiturates, tetrahydrocannabinol) appear in the urine over longer periods of time: several days in most cases, 1–2 months in chronic marijuana users. Sedative drug determinations are quite variable, amount of drug and duration of use being important determinants. False-positives can be a problem related to ingestion of some legitimate medications (eg, phenytoin for barbiturates, phenylpropanolamine for amphetamines, chlorpromazine for opioids) and some foods (eg, poppy seeds for opioids, coca leaf tea for cocaine). Manipulations can alter the legitimacy of the testing. Dilution, either in vivo or in vitro, can be detected by checking urine-specific gravity. Addition of ammonia, vinegar, or salt may invalidate the test, but odor and pH determinations are simple. Hair analysis can determine drug use over longer periods, particularly sequential drug-taking patterns. The sensitivity and reliability of such tests are considered good, and the method may be complementary to urinalysis.
O’Connor EA et al. Screening and behavioral counseling interventions to reduce unhealthy alcohol use in adolescents and adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2018 Nov 13;320(18):1910–8. [PMID: 30422198]
ALCOHOL USE DISORDER (Alcoholism)
Meshell D. Johnson, MD
ESSENTIALS OF DIAGNOSIS

 Physiologic dependence as manifested by evidence of withdrawal when intake is interrupted.
Physiologic dependence as manifested by evidence of withdrawal when intake is interrupted.
 Tolerance to the effects of alcohol.
Tolerance to the effects of alcohol.
 Evidence of alcohol-associated illnesses, such as alcoholic liver disease, cerebellar degeneration.
Evidence of alcohol-associated illnesses, such as alcoholic liver disease, cerebellar degeneration.
 Continued drinking despite strong medical and social contraindications and life disruptions.
Continued drinking despite strong medical and social contraindications and life disruptions.
 Impairment in social and occupational functioning.
Impairment in social and occupational functioning.
 Depression.
Depression.
 Blackouts.
Blackouts.
 General Considerations
General Considerations
Alcohol use disorder is a syndrome consisting of two phases: at-risk drinking and moderate to severe alcohol misuse. At-risk drinking is the repetitive use of alcohol, often to alleviate anxiety or solve other emotional problems. A moderate to severe alcohol use disorder is similar to that which occurs following the repeated use of other sedative-hypnotics and is characterized by recurrent use of alcohol despite disruption in social roles (family and work), alcohol-related legal problems, and taking safety risks by oneself and with others. The National Institute on Alcohol Abuse and Alcoholism formally defines at-risk drinking as more than 4 drinks per day or 14 drinks per week for men or more than 3 drinks per day or 7 drinks per week for women. A drink is defined by the Centers for Disease Control and Prevention (CDC) as 12 oz of beer, 8 oz of malt liquor, 5 oz of wine, or 1.3 oz or a “shot” of 80-proof distilled spirits of liquor. Individuals with at-risk drinking are at an increased risk for developing or are developing an alcohol use disorder. Alcohol and other drug abuse patients have a much higher prevalence of lifetime psychiatric disorders. While male-to-female ratios in alcoholic treatment agencies remain at 4:1, there is evidence that the rates are converging. Women delay seeking help, and when they do, they tend to seek it in medical or mental health settings. Adoption and twin studies indicate some genetic influence. Ethnic distinctions are important—eg, 40% of Japanese have aldehyde dehydrogenase deficiency and are more susceptible to the effects of alcohol. Depression is often present and should be evaluated carefully. The majority of suicides and intrafamily homicides involve alcohol. Alcohol is a major factor in rapes and other assaults.
There are several screening instruments that may help identify an alcohol use disorder. One of the most useful is the Alcohol Use Disorder Identification Test (AUDIT) (see Table 1–6).
 Clinical Findings
Clinical Findings
A. Acute Intoxication
The signs of alcoholic intoxication are the same as those of overdosage with any other central nervous system depressant: drowsiness, errors of commission, psychomotor dysfunction, disinhibition, dysarthria, ataxia, and nystagmus. For a 70-kg person, an ounce of whiskey, a 4- to 6-oz glass of wine, or a 12-oz bottle of beer (roughly 15, 11, and 13 grams of alcohol, respectively) may raise the level of alcohol in the blood by 25 mg/dL. For a 50-kg person, the blood alcohol level would rise even higher (35 mg/dL) with the same consumption. Blood alcohol levels below 50 mg/dL rarely cause significant motor dysfunction (the legal limit for driving under the influence is commonly 80 mg/dL). Intoxication as manifested by ataxia, dysarthria, and nausea and vomiting indicates a blood level greater than 150 mg/dL, and lethal blood levels range from 350 mg/dL to 900 mg/dL. In severe cases, overdosage is marked by respiratory depression, stupor, seizures, shock syndrome, coma, and death. Serious overdoses are frequently due to a combination of alcohol with other sedatives.
B. Withdrawal
There is a wide spectrum of manifestations of alcohol withdrawal, ranging from anxiety, decreased cognition, and tremulousness, through increasing irritability and hyperreactivity to full-blown delirium tremens (DTs). Alcohol withdrawal syndrome can be categorized as mild, moderate, or severe withdrawal, withdrawal seizures, and DTs. Symptoms of mild withdrawal, including tremor, anxiety, tachycardia, nausea, vomiting, and insomnia, begin within 6 hours after the last drink, often before the blood alcohol levels drop to zero, and usually have passed by day 2. Severe or major withdrawal occurs 48–96 hours after the last drink and is usually preceded by prolonged heavy alcohol use. Symptoms include disorientation, agitation, diaphoresis, whole body tremor, vomiting, hypertension, and hallucinations (visual > tactile > auditory). Moderate withdrawal symptoms and signs fall between those of minor and major withdrawal. Withdrawal seizures can occur as early as 8 hours after the last drink but usually do not manifest more than 48 hours after alcohol cessation. Seizures are more prevalent in persons who have a history of withdrawal syndromes. These seizures are generalized tonic-clonic seizures, are brief in duration, and resolve spontaneously. If withdrawal is untreated, these seizures can recur in about 60% of patients. DTs will develop in approximately half of these patients. If seizures are focal, associated with trauma or fever, or have an onset more than 48 hours after the last drink, another etiology for seizures must be considered. DT is the most severe form of alcohol withdrawal. It is an acute organic psychosis that usually manifests 48–72 hours after the last drink, but may occur up to 7–10 days later. It is characterized by extreme mental confusion, agitation, tremor, diaphoresis, sensory hyperacuity, visual hallucinations (often of snakes, bugs, etc), and autonomic hyperactivity (tachycardia and hypertension). Complications of DTs include (1) dehydration, (2) electrolyte disturbances (hypokalemia, hypomagnesemia), (3) arrhythmias and seizures, and (4) cardiovascular collapse and death. The acute withdrawal syndrome is often unexpected, occurring when the patient has been hospitalized for an unrelated problem, thus presenting as a diagnostic dilemma. Suspect alcohol withdrawal in every unexplained delirium. The mortality rate from DTs, which was upward of 35%, has steadily decreased with early diagnosis and improved treatment.
In addition to the immediate withdrawal symptoms, there is evidence of persistent longer-term ones, including sleep disturbances, anxiety, depression, excitability, fatigue, and emotional volatility. These symptoms may persist for 3–12 months, and in some cases they become chronic.
C. Alcoholic (Organic) Hallucinosis
This syndrome occurs either during heavy drinking or on withdrawal and is characterized by a paranoid psychosis without the tremulousness, confusion, and clouded sensorium seen in withdrawal syndromes. The patient appears normal except for the auditory hallucinations, which are frequently persecutory and may cause the patient to behave aggressively and in a paranoid fashion.
D. Chronic Alcoholic Brain Syndromes
These encephalopathies are characterized by increasing erratic behavior, memory and recall problems, and emotional instability—the usual signs of organic brain injury due to any cause. Wernicke-Korsakoff syndrome due to thiamine deficiency may develop with a series of episodes. Wernicke encephalopathy consists of the triad of confusion, ataxia, and ophthalmoplegia (typically sixth nerve palsy). Early recognition and treatment with thiamine can minimize damage. One of the possible sequelae is Korsakoff psychosis, characterized by both anterograde and retrograde amnesia, with confabulation early in the course. Early recognition and treatment with intravenous thiamine and B complex vitamins can minimize damage. Excessive alcohol consumption in men has been associated with faster cognitive decline compared with light to moderate alcohol consumption.
E. Laboratory Findings
Ethanol may contribute to the presence of an otherwise unexplained osmolar gap. There may also be increased serum liver biochemical tests, uric acid, and triglycerides and decreased serum potassium and magnesium. The most definitive biologic marker for chronic alcoholism is carbohydrate deficient transferrin, which can detect heavy use (60 mg/day over 7–10 days) with high specificity. Other useful tests for diagnosing alcohol use disorder are gamma-glutamyl transpeptidase (GGT) measurement (levels greater than 30 units/L are suggestive of heavy drinking) and mean corpuscular volume (MCV) (more than 95 fL in men and more than 100 fL in women). If both are elevated, a serious alcohol problem is likely. Use of other recreational drugs with alcohol skews and negates the significance of these tests.
 Differential Diagnosis
Differential Diagnosis
The differential diagnosis of alcoholism is essentially between primary alcohol use disorder (when no other major psychiatric diagnosis exists) and secondary alcohol use disorder (when alcohol is used as self-medication for major underlying psychiatric problems such as schizophrenia or affective disorder). The differentiation is important, since the latter group requires treatment for the specific psychiatric problem. In primary and secondary alcoholism, at-risk drinking can be distinguished from alcohol addiction by taking a careful psychiatric history and evaluating the degree to which recurrent drinking impacts the social role functioning and physical safety of the individual.
The differential diagnosis of alcohol withdrawal includes other sedative withdrawals and other causes of delirium. Acute alcoholic hallucinosis must be differentiated from other acute paranoid states such as amphetamine psychosis or paranoid schizophrenia. The form of the brain syndrome is of little help—eg, chronic brain syndromes from systemic lupus erythematosus may be associated with confabulation similar to that resulting from longstanding alcoholism.
 Complications
Complications
The medical, economic, and psychosocial problems of alcoholism are staggering. The central and peripheral nervous system complications include chronic brain syndromes, cerebellar degeneration, cardiomyopathy, and peripheral neuropathies. Direct effects on the liver include cirrhosis, esophageal varices, and eventual hepatic failure. Indirect effects include protein abnormalities, coagulation defects, hormone deficiencies, and an increased incidence of liver neoplasms.
Fetal alcohol syndrome includes one or more of the following developmental defects in the offspring of alcoholic women: (1) low birth weight and small size with failure to catch up in size or weight, (2) mental retardation, with an average IQ in the 60s, and (3) a variety of birth defects, with a large percentage of facial and cardiac abnormalities. The risk is appreciably higher with the more alcohol ingested by the mother each day.
 Treatment of At-Risk Drinking
Treatment of At-Risk Drinking
A. Psychological
The most important consideration for the clinician is to suspect the problem early and take a nonjudgmental attitude, although this does not mean a passive one. The problem of denial must be faced, preferably with significant family members at the first meeting. This means dealing from the beginning with any enabling behavior of the spouse or other significant people. Enabling behavior allows the patient with an alcohol use disorder to avoid facing the consequences of his or her behavior.
There must be an emphasis on the things that can be done. This approach emphasizes the fact that the clinician cares and strikes a positive and hopeful note early in treatment. Valuable time should not be wasted trying to find out why the patient drinks; come to grips early with the immediate problem of how to stop the drinking. Although total abstinence should be the ultimate goal, a harm reduction model indicates that gradual progress toward abstinence can be a useful treatment strategy.
Motivational interviewing, a model of counseling that addresses both the patient’s ambivalence and motivation for change, may contribute to reduced consumption over time.
B. Social
Encourage the patient to attend Alcoholics Anonymous meetings and the spouse to attend Al-Anon meetings. Success is usually proportionate to the utilization of Alcoholics Anonymous, religious counseling, and other resources. The patient should be seen frequently for short periods.
Do not underestimate the importance of religion, particularly since the patient with alcohol use disorder is often a dependent person who needs a great deal of support. Early enlistment of the help of a concerned religious adviser can often provide the turning point for a personal conversion to sobriety.
One of the most important considerations is the patient’s job—fear of losing a job is one of the most powerful motivations for giving up alcohol. The business community is aware of the problem; about 70% of the Fortune 500 companies offer programs to their employees to help with the problem of alcoholism. Some specific recommendations that can be offered to employers include (1) avoid placement in jobs where the alcoholic patient must be alone, eg, as a traveling buyer or sales executive, (2) use supervision but not surveillance, (3) keep competition with others to a minimum, and (4) avoid positions that require quick decision making on important matters (high-stress situations). In general, commitment to abstinence and avoidance of situations that might be conducive to drinking are most predictive of a good outcome.
C. Medical
Hospitalization is not usually necessary but may be warranted if there are concomitant medical indications. Furthermore, if patients with heavy alcohol use are hospitalized for any other reason, providers must be vigilant for signs and symptoms of alcohol withdrawal.
Because of the many medical complications of alcoholism, a complete physical examination with appropriate laboratory tests is mandatory, with special attention to the liver and nervous system. Use of sedatives as a replacement for alcohol is not desirable. Lithium is not helpful in the treatment of alcoholism.
Disulfiram (250–500 mg/day orally) has been used for many years as an aversive medication to discourage alcohol use. Disulfiram inhibits aldehyde dehydrogenase, causing toxic reactions when alcohol is consumed. The results have generally been of limited effectiveness and depend on the motivation of the individual to be compliant.
Naltrexone, an opiate antagonist, in a dosage of 50 mg orally daily, lowers relapse rates over the 3–6 months after cessation of drinking, apparently by lessening the pleasurable effects of alcohol. One study suggests that naltrexone is most effective when given during periods of drinking in combination with therapy that supports abstinence but accepts the fact that relapses occur. Naltrexone is FDA approved for maintenance therapy. Studies indicate that it reduces alcohol craving when used as part of a comprehensive treatment program. Acamprosate (333–666 mg orally three times daily) helps reduce craving and maintain abstinence and can be continued even during periods of relapse. Both acamprosate and oral naltrexone have been associated with reduction in return to drinking.
D. Behavioral
Conditioning approaches historically have been used in some settings in the treatment of alcoholism, most commonly as a type of aversion therapy. For example, the patient is given a drink of whiskey and then a shot of apomorphine, and proceeds to vomit. In this way a strong association is built up between the drinking and vomiting. Although this kind of treatment has been successful in some cases, after appropriate informed consent, many people do not sustain the learned aversive response.
 Treatment of Hallucinosis & Withdrawal
Treatment of Hallucinosis & Withdrawal
A. Hallucinosis
Alcoholic hallucinosis, which can occur either during or on cessation of a prolonged drinking period, is not a typical withdrawal syndrome and is handled differently. Since the symptoms are primarily those of a psychosis in the presence of a clear sensorium, they are handled like any other psychosis: hospitalization (when indicated) and adequate amounts of antipsychotic medications. Haloperidol, 5 mg orally twice a day for the first day or so, usually ameliorates symptoms quickly, and the medication can be decreased and discontinued over several days as the patient improves. It then becomes necessary to deal with the chronic alcohol abuse, which has been discussed.
B. Withdrawal
The onset of withdrawal symptoms is usually 6–36 hours, and the peak intensity of symptoms is 48–72 hours after alcohol consumption is stopped. Providing adequate central nervous system depressants (eg, benzodiazepines) is important to counteract the excitability resulting from sudden cessation of alcohol intake. The choice of a specific sedative is less important than using adequate doses to bring the patient to a level of moderate sedation, and this will vary from person to person.
All patients should be evaluated for their risk of alcohol withdrawal. Mild dependency requires “drying out.” For outpatients, in some instances, a short course of tapering long-acting benzodiazepines—eg, diazepam, 20 mg/day orally initially, decreasing by 5 mg daily—may be a useful adjunct. When the history or presentation suggests that patients are actively in withdrawal or at significant risk for withdrawal, they should be hospitalized. Risk factors include a recent drinking history, frequent alcohol consumption, a past history of withdrawal, seizures, hallucinosis, or DTs, a past history of needing medication for detoxification, or a history of benzodiazepine or barbiturate use, abuse, or dependency.
For all hospitalized patients, general management includes ensuring adequate hydration, correction of electrolyte imbalances (particularly magnesium, calcium, and potassium), and administering the vitamins thiamine (100 mg intravenously daily for 3 days then orally daily), folic acid (1 mg orally daily), and a multivitamin orally daily. Thiamine should be given prior to any glucose-containing solutions to decrease the risk of precipitating Wernicke encephalopathy or Korsakoff syndrome. Alcohol withdrawal is treated with benzodiazepines. Continual assessment is recommended to determine the severity of withdrawal, and symptom-driven medication regimens, which have been shown to prevent undersedation as well as oversedation and to reduce total benzodiazepine usage over fixed-dose schedules, should be used. The severity of withdrawal will determine a patient’s level of care. For those at risk for withdrawal and with mild withdrawal symptoms, admission to a medical unit is adequate. For those with moderate withdrawal, a higher acuity hospital environment is recommended. Those with severe withdrawal should be admitted to the ICU.
1. Assessing alcohol withdrawal symptom severity—The Clinical Institute Withdrawal Assessment for Alcohol, Revised (CIWA-Ar) is a validated tool that is widely used to determine severity of alcohol withdrawal. This survey assesses symptoms in 10 areas and can be administered relatively quickly (Figure 25–3). One caveat is that the patient must be able to communicate his or her symptoms to the provider. The maximum attainable score is 67. Clinical judgment should be used to determine final dosing of medications to patients who are in alcohol withdrawal because dosing will vary between patients and degrees of withdrawal.
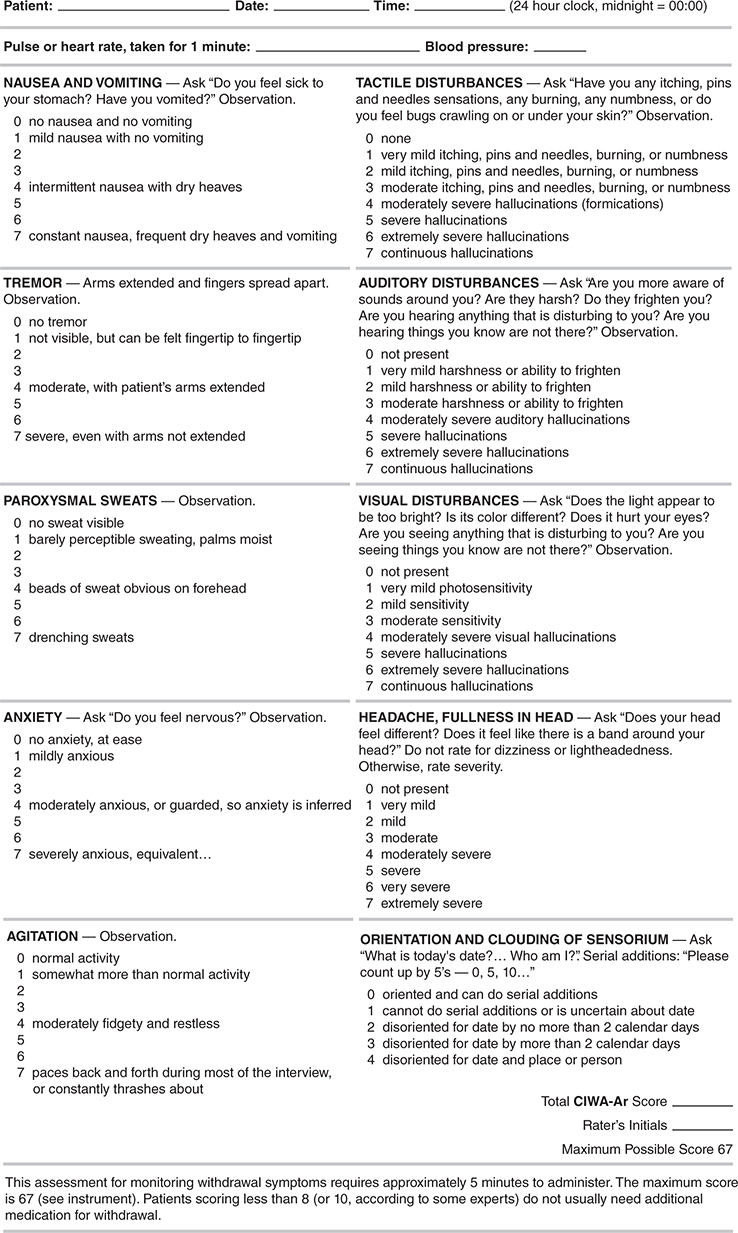
Figure 25–3. Alcohol withdrawal assessment. (Reproduced from Sullivan JT et al. Assessment of alcohol withdrawal: The revised clinical institute withdrawal assessment for alcohol scale [CIWA-Ar]. Br J Addict 1989;84:1353. This scale is not copyrighted and may be used freely.)
2. Treating alcohol withdrawal symptoms based on CIWA-Ar score—
a. Minimal withdrawal symptoms (CIWA-Ar score less than 8)—Patients who have a history suggestive of alcohol withdrawal risk with minimal withdrawal symptoms are suitable for withdrawal prophylaxis. The recommended benzodiazepine options include chlordiazepoxide or lorazepam orally, tapered over 3 days. The protocol calls for nursing assessment of sedation and withdrawal symptoms (CIWA-Ar) every 6 hours. If prophylactic medication is indicated, a sample tapering regimen may include lorazepam, 1 mg orally every 6 hours for 1 day, then 1 mg orally every 8 hours for 1 day, then 1 mg orally every 12 hours for 1 day, then discontinue; or chlordiazepoxide, 50 mg orally every 6 hours for 1 day, 25 mg orally every 6 hours for 2 days, then discontinue. Avoid chlordiazepoxide in the elderly or in patients with liver disease. Lorazepam is preferred in patients with liver disease. Sedation is assessed 30–60 minutes after each medication dose. The benzodiazepine dose is held for oversedation or if the respiratory rate is less than 10 breaths per minute. For CIWA-Ar score greater than 8, the provider must be notified, because this is suggestive of active withdrawal, and escalation of treatment must occur.
b. Mild withdrawal symptoms (CIWA-Ar score 8–15)—For patients in mild withdrawal, either chlordiazepoxide orally or lorazepam orally or intravenously can be used. Initially, chlordiazepoxide 50 mg orally or lorazepam 1 or 2 mg orally or intravenously is given hourly for 2 hours. Patients must be assessed for level of sedation and withdrawal symptoms (CIWA-Ar) every 4 hours. Dosing is adjusted as necessary to control symptoms without excessive sedation. After the first 2 hours, chlordiazepoxide or lorazepam is given every 4 hours and as needed. Typical dosing may include chlordiazepoxide 25–50 mg orally or lorazepam 0.5–1 mg orally or intravenously every 4 hours as needed. Additional doses of benzodiazepines should be given if the CIWA-Ar score remains between 8 and 15.
c. Moderate withdrawal (CIWA-Ar score 16–20)—For patients in moderate withdrawal, chlordiazepoxide 100 mg orally or lorazepam 3 or 4 mg orally or intravenously is given every hour for the first 2 hours. CIWA-Ar monitoring should occur every 2 hours. Dosing is adjusted to control symptoms without excessive sedation. After initial dosing, continued treatment could include chlordiazepoxide 50 mg orally or lorazepam 1–2 mg orally or intravenously every 2 hours as needed for CIWA-Ar score between 16 and 20, and chlordiazepoxide 25 mg orally or lorazepam 0.5–1 mg orally or intravenously every 2 hours for CIWA-Ar score between 8 and 15. The maximum dose of chlordiazepoxide is 600 mg in 24 hours. Continuous pulse oximetry and cardiac monitoring should be considered. The degree of sedation should be monitored 30–60 minutes after each oral dose of medication and for 15 minutes after each parenteral dose.
d. Severe withdrawal (CIWA-Ar score greater than 20)—Patients with severe withdrawal are at risk for the development of DTs and should be transferred or admitted to the ICU. Intravenous lorazepam can be used to treat severe withdrawal. A potential treatment protocol can administer lorazepam 1–2 mg intravenously every 15 minutes until the patient is calm and sedated but awake. Initial CIWA-Ar monitoring should occur every 30 minutes. The patient can then receive lorazepam 2 mg orally or intravenously every hour as needed when the CIWA-Ar score is between 16 and 20, and lorazepam 1–2 mg orally or intravenously every hour as needed when the CIWA-Ar score is between 8 and 15. If the patient requires more than 8 mg/h of lorazepam as an initial dose or continues to demonstrate observable agitation, tremors, tachycardia, or hypertension despite high doses of lorazepam, consider adding dexmedetomidine. Dexmedetomidine, an alpha-2-agonist, produces sedation with minimal effect on respiratory drive. It is not recommended as a sole agent for the treatment of alcohol withdrawal but as adjunctive therapy along with benzodiazepines to decrease the hyperadrenergic output in patients with severe alcohol withdrawal not controlled by benzodiazepines or in patients at risk for respiratory depression from high-dose benzodiazepine administration. The recommended dosing of dexmedetomidine is 0.2–0.7 mcg/kg/h, with lorazepam 1–2 mg intravenously every 8 hours plus lorazepam 1–2 mg intravenously every hour as needed for agitation. In limited cases of severe withdrawal requiring frequent lorazepam boluses for at least 6 hours, continuous intravenous lorazepam infusion can be considered, but the patient must be monitored extremely carefully for signs of respiratory depression. Continuous pulse oximetry and close observance of the patient’s respiratory status is required. Sedation is assessed 15 minutes after each intravenous dose. If withdrawal symptoms are refractory to escalating benzodiazepine usage, despite the addition of dexmedetomidine, escalation to propofol should be considered. Patients receiving large doses of benzodiazepines often require intubation for airway protection, at which time initiation of propofol infusion for sedation, in addition to treatment of refractory alcohol withdrawal, is recommended. Phenobarbital monotherapy for alcohol withdrawal is used at some institutions, but randomized controlled trials comparing the efficacy of phenobarbital over benzodiazepines are needed to inform adoption of new treatment regimens.
In all cases, benzodiazepines should be held if the patient is too sedated or has a respiratory rate less than 10 breaths per minute. Do not bolus lorazepam in doses greater than 4 mg intravenously. Mixing benzodiazepines, eg, chlordiazepoxide orally every 8 hours with lorazepam, is not recommended. Instead, select a single agent and titrate as needed. Once a patient has been stable for 24 hours, the benzodiazepine dose can be reduced by 20% daily until withdrawal is complete.
3. Managing other withdrawal-associated conditions—Meticulous examination for other medical problems is necessary. Alcoholic hypoglycemia can occur with low blood alcohol levels (see Chapter 27). Patients with severe alcohol use disorder commonly have liver disease with associated clotting disorders and are also prone to injury—and the combination all too frequently leads to undiagnosed subdural hematoma.
Phenytoin does not appear to be useful in managing alcohol withdrawal seizures per se. Sedating doses of benzodiazepines are effective in treating alcohol withdrawal seizures. Thus, other anticonvulsants are not usually needed unless there is a preexisting seizure disorder.
Chronic brain syndromes secondary to a long history of alcohol intake are not clearly responsive to thiamine and vitamin replenishment. Attention to the social and environmental care of this type of patient is paramount.
4. Initiating psychological and social measures—The psychological and behavioral treatment methods outlined under Treatment of At-Risk Drinking become the primary considerations after successful treatment of alcoholic hallucinosis or withdrawal. Psychological and social measures should be initiated in the hospital prior to discharge. This increases the possibility of continued posthospitalization treatment.
Oks M et al. The safety and utility of phenobarbital use for the treatment of severe alcohol withdrawal syndrome in the medical intensive care unit. J Intensive Care Med. 2018 Jan 1:885066618783947. [PMID: 29925291]
Sullivan SM et al. Comparison of phenobarbital-adjunct versus benzodiazepine-only approach for alcohol withdrawal syndrome in the emergency department. Am J Emerg Med. 2019 Jul;37(7):1313–6. [PMID: 30414743]
Soyka M et al; WFSBP Task Force on Treatment Guidelines for Substance Use Disorders. Guidelines for biological treatment of substance use and related disorders, part 1: Alcoholism, first revision. World J Biol Psychiatry. 2017 Mar;18(2):86–119. [PMID: 28006997]
OTHER DRUG & SUBSTANCE USE DISORDERS
1. Opioids
While the terms “opioids” and “narcotics” both refer to a group of drugs with actions that mimic those of morphine, the term “opioids” is used when discussing medications prescribed in a controlled manner by a clinician, and the term “narcotics” is used to connote illicit drug use. The opioid analgesics can be reversed by the opioid antagonist naloxone.
The clinical symptoms and signs of mild narcotic intoxication include changes in mood, with feelings of euphoria; drowsiness; nausea with occasional emesis; needle tracks; and miosis. The incidence of snorting and inhaling (“smoking”) heroin is increasing, particularly among cocaine users. This coincides with a decrease in the availability of methaqualone (no longer marketed) and other sedatives used to temper the cocaine “high” (see discussion of cocaine under Stimulants, below). Overdosage causes respiratory depression, peripheral vasodilation, pinpoint pupils, pulmonary edema, coma, and death.
Tolerance and withdrawal are major concerns when continued use of opioids occurs, although withdrawal causes only moderate morbidity (similar in severity to a bout of “flu”). Grades of withdrawal are categorized from 0 to 4: grade 0 includes craving and anxiety; grade 1, yawning, lacrimation, rhinorrhea, and perspiration; grade 2, previous symptoms plus mydriasis, piloerection, anorexia, tremors, and hot and cold flashes with generalized aching; grades 3 and 4, increased intensity of previous symptoms and signs, with increased temperature, blood pressure, pulse, and respiratory rate and depth. In withdrawal from the most severe addiction, vomiting, diarrhea, weight loss, hemoconcentration, and spontaneous ejaculation or orgasm commonly occur.
Treatment for overdosage (or suspected overdosage) is discussed in Chapter 38.
Treatment for withdrawal begins if grade 2 signs develop. If a withdrawal program is necessary, use methadone, 10 mg orally (use parenteral administration if the patient is vomiting), and observe. If signs (piloerection, mydriasis, cardiovascular changes) persist for more than 4–6 hours, give another 10 mg; continue to administer methadone at 4- to 6-hour intervals until signs are not present (rarely greater than 40 mg of methadone in 24 hours). Divide the total amount of medication required over the first 24-hour period by 2 and give that amount every 12 hours. Each day, reduce the total 24-hour dose by 5–10 mg. Thus, a moderately addicted patient initially requiring 30–40 mg of methadone could be withdrawn over a 4- to 8-day period. Clonidine, 0.1 mg orally several times daily over a 10- to 14-day period, is both an alternative and an adjunct to methadone detoxification; it is not necessary to taper the dose. Clonidine is helpful in alleviating cardiovascular symptoms but does not significantly relieve anxiety, insomnia, or generalized aching. There is a protracted abstinence syndrome of metabolic, respiratory, and blood pressure changes over a period of 3–6 months. Alternative strategies for the treatment of opioid withdrawal have included rapid and ultrarapid detoxification techniques. However, data do not support the use of either method.
Treatment of opioid use disorder is key given evidence of significant morbidity and mortality, including what has been called the “opioid epidemic” in the United States. Opioid antagonists (eg, naltrexone) can also be used successfully for treatment of the patient who has been free of opioids for 7–10 days. Naltrexone blocks the narcotic “high” of heroin when 50 mg is given orally every 24 hours initially for several days and then 100 mg is given every 48–72 hours. A monthly injectable form of naltrexone is available and may enhance compliance. Liver disorders are a major contraindication. Buprenorphine, a partial agonist, is a mainstay of office-based treatment of opiate dependency. Its use requires certified training along with a special license from the Drug Enforcement Agency. Buprenorphine is a mu partial agonist and kappa antagonist. Unlike conventional opioids, buprenorphine may have a role in the treatment of major depression.
Methadone maintenance programs are of some value in chronic recidivism. Under carefully controlled supervision, the narcotic addict is maintained on fairly high doses of methadone (40–120 mg/day) that satisfy craving and block the effects of heroin to a great degree.
Bohnert ASB et al. Understanding links among opioid use, overdose, and suicide. N Engl J Med. 2019 Jan 3;380(1):71–9. [PMID: 30601750]
Jones CM et al. Naloxone co-prescribing to patients receiving prescription opioids in the Medicare Part D program, United States, 2016–2017. JAMA. 2019 Aug 6;322(5):462–4. [PMID: 31386124]
Leshner AI et al. Medication-based treatment to address opioid use disorder. JAMA. 2019 Jun 4;321(21):2071–2. [PMID: 31046072]
National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Sciences Policy; Committee on Medication-Assisted Treatment for Opioid Use Disorder; Mancher M et al (editors). Medications for Opioid Use Disorder Save Lives. Washington (DC): National Academies Press (US), 2019. [PMID: 30896911]
Olfson M et al. Trends in intentional and unintentional opioid overdose deaths in the United States, 2000–2017. JAMA. 2019 Dec 17;322(23):2340–2. [PMID: 31846008]
2. Sedatives (Anxiolytics)
See Anxiety Disorders, this chapter.
3. Psychedelics
All of the common psychedelics (LSD, mescaline, psilocybin, dimethyltryptamine, and other derivatives of phenylalanine and tryptophan) can produce similar behavioral and physiologic effects. An initial feeling of tension is followed by emotional release such as crying or laughing (1–2 hours). Later and at higher doses, perceptual distortions occur, with visual illusions and hallucinations, and occasionally there is fear of ego disintegration (2–3 hours). Major changes in time sense and mood lability then occur (3–4 hours). A feeling of detachment and a sense of destiny and control occur (4–6 hours). Of course, reactions vary among individuals, and some of the drugs produce markedly different time frames. Occasionally, the acute episode is terrifying (a “bad trip”), which may include panic, depression, confusion, or psychotic symptoms. Preexisting emotional problems, the attitude of the user, and the setting where the drug is used affect the experience.
Treatment of the acute episode primarily involves protection of the individual from erratic behavior that may lead to injury or death. A structured environment is usually sufficient until the drug is metabolized. In severe cases, antipsychotic medications with minimal side effects (eg, haloperidol, 5 mg intramuscularly) may be given every several hours until the individual has regained control. In cases where “flashbacks” occur (mental imagery from a “bad trip” that is later triggered by mild stimuli such as marijuana, alcohol, or psychic trauma), a short course of an antipsychotic medication—eg, olanzapine, 5–10 mg/day orally, or risperidone, 2 mg/day orally, initially, and up to 20 mg/day and 6 mg/day, respectively—is usually sufficient. Lorazepam or clonazepam, 1–2 mg orally every 2 hours as needed for acute agitation, may be a useful adjunct. An occasional patient may have “flashbacks” for much longer periods and may require small doses of antipsychotic medications over the longer term.
4. Phencyclidine
Phencyclidine (PCP, angel dust, peace pill, hog) is simple to produce and mimics to some degree the traditional psychedelic drugs. PCP is a common deceptive substitute for LSD, tetrahydrocannabinol, and mescaline. It is available in crystals, capsules, and tablets to be inhaled, injected, swallowed, or smoked (it is commonly sprinkled on marijuana).
Absorption after smoking is rapid, with onset of symptoms in several minutes and peak symptoms in 15–30 minutes. Mild intoxication produces euphoria accompanied by a feeling of numbness. Moderate intoxication (5–10 mg) results in disorientation, detachment from surroundings, distortion of body image, combativeness, unusual feats of strength (partly due to its anesthetic activity), and loss of ability to integrate sensory input, especially touch and proprioception. Physical symptoms include dizziness, ataxia, dysarthria, nystagmus, retracted upper eyelid with blank stare, hyperreflexia, and tachycardia. There are increases in blood pressure, respiration, muscle tone, and urine production. Usage in the first trimester of pregnancy is associated with an increase in spontaneous abortion and congenital defects. Severe intoxication (20 mg or more) produces an increase in degree of moderate symptoms, with the addition of seizures, deepening coma, hypertensive crisis, and severe psychotic ideation. The drug is particularly long-lasting (several days to several weeks) owing to high lipid solubility, gastroenteric recycling, and the production of active metabolites. Overdosage may be fatal, with the major causes of death being hypertensive crisis, respiratory arrest, and convulsions. Acute rhabdomyolysis has been reported and can result in myoglobinuric kidney failure.
Differential diagnosis involves the whole spectrum of street drugs, since in some ways phencyclidine mimics sedatives, psychedelics, and marijuana in its effects. Blood and urine testing can detect the acute problem.
Treatment is discussed in Chapter 38.
5. Marijuana
Cannabis sativa, a hemp plant, is the source of marijuana. The drug is usually inhaled by smoking but vaporizing is increasingly popular. There is emerging evidence that there is a clinically distinct syndrome associated with “vaping” THC—vaping-associated lung injury—that may result in devastating pulmonary effects and a pathologically distinct pathophysiology. Effects occur in 10–20 minutes and last 2–3 hours. “Joints” of good quality contain about 500 mg of marijuana (which contains approximately 5–15 mg of tetrahydrocannabinol with a half-life of 7 days).
With moderate dosage, recreational marijuana (higher in the THC versus CBD component) produces two phases: mild euphoria followed by sleepiness. In the acute state, the user has an altered time perception, less inhibited emotions, psychomotor problems, impaired immediate memory, and conjunctival injection. High doses produce transient psychotomimetic effects. No specific treatment is necessary except in the case of the occasional “bad trip,” in which case the person is treated in the same way as for psychedelic usage. Marijuana frequently aggravates existing mental illness and adversely affects motor performance.
Studies of long-term effects have conclusively shown abnormalities in the pulmonary tree. Laryngitis and rhinitis are related to prolonged use, along with chronic obstructive pulmonary disease. Electrocardiographic abnormalities are common, but no chronic cardiac disease has been linked to marijuana use. Long-term usage has resulted in depression of plasma testosterone levels and reduced sperm counts. Abnormal menstruation and failure to ovulate have occurred in some women. Cognitive impairments are common. Health care utilization for a variety of health problems is increased in long-term marijuana smokers. Sudden withdrawal produces insomnia, nausea, myalgia, and irritability. Psychological effects of long-term marijuana usage are still unclear. Urine testing is reliable if samples are carefully collected and tested. Detection periods span 4–6 days in short-term users and 20–50 days in long-term users.
6. Stimulants: Amphetamines & Cocaine
Stimulant abuse is quite common, either alone or in combination with abuse of other drugs. The stimulants include illicit drugs such as methamphetamine (“speed”)—one variant is a smokable form called “ice,” which gives an intense and fairly long-lasting high—and methylphenidate and dextroamphetamine, which are under prescription control. Street availability of amphetamines remains high. Moderate usage of any of the stimulants produces hyperactivity, a sense of enhanced physical and mental capacity, and sympathomimetic effects. The clinical picture of acute stimulant intoxication includes sweating, tachycardia, elevated blood pressure, mydriasis, hyperactivity, and an acute brain syndrome with confusion and disorientation. Tolerance develops quickly, and, as the dosage is increased, hypervigilance, paranoid ideation (with delusions of parasitosis), stereotypy, bruxism, tactile hallucinations of insect infestation, and full-blown psychoses occur, often with persecutory ideation and aggressive responses. Stimulant withdrawal is characterized by depression with symptoms of hyperphagia and hypersomnia.
People who have used stimulants chronically (eg, anorexigenics) occasionally become sensitized (“kindling”) to future use of stimulants. In these individuals, even small amounts of mild stimulants such as caffeine can cause symptoms of paranoia and auditory hallucinations.
Cocaine is a stimulant. It is a product of the coca plant. The derivatives include seeds, leaves, coca paste, cocaine hydrochloride, and the free base of cocaine. Cocaine hydrochloride is the salt and the most commonly used form. Freebase, a purer (and stronger) derivative called “crack,” is prepared by simple extraction from cocaine hydrochloride.
There are various modes of use. Coca leaf chewing involves toasting the leaves and chewing with alkaline material (eg, the ash of other burned leaves) to enhance buccal absorption. One achieves a mild high, with onset in 5–10 minutes and lasting for about an hour. Intranasal use is simply snorting cocaine through a straw. Absorption is slowed somewhat by vasoconstriction (which may eventually cause tissue necrosis and septal perforation); the onset of action is in 2–3 minutes, with a moderate high (euphoria, excitement, increased energy) lasting about 30 minutes. The purity of the cocaine is a major determinant of the high. Intravenous use of cocaine hydrochloride or “freebase” is effective in 30 seconds and produces a short-lasting, fairly intense high of about 15 minutes’ duration. The combined use of cocaine and ethanol results in the metabolic production of cocaethylene by the liver. This substance produces more intense and long-lasting cocaine-like effects. Smoking freebase (volatilized cocaine because of the lower boiling point) acts in seconds and results in an intense high lasting several minutes. The intensity of the reaction is related to the marked lipid solubility of the freebase form and produces by far the most severe medical and psychiatric symptoms.
Cardiovascular collapse, arrhythmias, myocardial infarction, and transient ischemic attacks have been reported. Seizures, strokes, migraine symptoms, hyperthermia, and lung damage may occur, and there are several obstetric complications, including spontaneous abortion, abruptio placentae, teratogenic effects, delayed fetal growth, and prematurity. Cocaine can cause anxiety, mood swings, and delirium, and chronic use can cause the same problems as other stimulants.
Clinicians should be alert to cocaine use in patients presenting with unexplained nasal bleeding or septal perforations, headaches, fatigue, insomnia, anxiety, depression, and chronic hoarseness. Sudden withdrawal of the drug is not life-threatening but usually produces craving, sleep disturbances, hyperphagia, lassitude, and severe depression (sometimes with suicidal ideation) lasting days to weeks.
Treatment is imprecise and difficult. Since the high is related to blockage of dopamine reuptake, the dopamine agonist bromocriptine, 1.5 mg orally three times a day, alleviates some of the symptoms of craving associated with acute cocaine withdrawal. Treatment of psychosis is the same as that of any psychosis: antipsychotic medications in dosages sufficient to alleviate the symptoms. Any medical symptoms (eg, hyperthermia, seizures, hypertension) are treated specifically. These approaches should be used in conjunction with a structured program, most often based on the Alcoholics Anonymous model. Hospitalization may be required if self-harm or violence toward others is a perceived threat (usually indicated by paranoid delusions).
7. Caffeine
Caffeine, along with nicotine and alcohol, is one of the most commonly used drugs worldwide. Tea, cocoa, and cola drinks also contribute to an intake of caffeine that is often astoundingly high in a large number of people. Low to moderate doses (30–200 mg/day) tend to improve some aspects of performance (eg, vigilance). The approximate content of caffeine in a (180-mL) cup of beverage is as follows: brewed coffee, 80–140 mg; instant coffee, 60–100 mg; decaffeinated coffee, 1–6 mg; black leaf tea, 30–80 mg; tea bags, 25–75 mg; instant tea, 30–60 mg; cocoa, 10–50 mg; and 12-oz cola drinks, 30–65 mg. A 2-oz chocolate candy bar has about 20 mg. Some herbal teas (eg, “morning thunder”) contain caffeine. Caffeine-containing analgesics usually contain approximately 30 mg per unit. Symptoms of caffeinism (usually associated with ingestion of over 500 mg/day) include anxiety, agitation, restlessness, insomnia, a feeling of being “wired,” and somatic symptoms referable to the heart and gastrointestinal tract. It is common for a case of caffeinism to present as an anxiety disorder. It is also common for caffeine and other stimulants to precipitate severe symptoms in compensated schizophrenic and manic-depressive patients. Chronically depressed patients often use caffeine drinks as self-medication. This diagnostic clue may help distinguish some major affective disorders. Discontinuation of caffeine (greater than 250 mg/day) can produce withdrawal symptoms, such as headaches, irritability, lethargy, and occasional nausea.
8. Miscellaneous Drugs & Solvents
The principal over-the-counter drugs of concern are an assortment of antihistaminic agents, frequently in combination with a mild analgesic promoted as cold remedies.
Antihistamines usually produce some central nervous system depression—thus their use as over-the-counter sedatives. Practically all of the so-called sleep aids are antihistamines. The mixture of antihistamines with alcohol usually exacerbates the central nervous system effects. Scopolamine and bromides generally have been removed from over-the-counter products.
The abuse of laxatives sometimes can lead to electrolyte disturbances that may contribute to the manifestations of a delirium. The greatest use of laxatives tends to be in the elderly and in those with eating disorders, both of whom are the most vulnerable to physiologic changes.
Anabolic steroids are abused by people who wish to increase muscle mass for cosmetic reasons or for greater strength. In addition to the medical problems, the practice is sometimes associated with significant mood swings, aggressiveness, and paranoid delusions. Alcohol and stimulant use are higher in these individuals. Withdrawal symptoms of steroid dependency include fatigue, depressed mood, restlessness, and insomnia.
Amyl nitrite is used as an “orgasm expander.” The changes in time perception, “rush,” and mild euphoria caused by the drug prompted its nonmedical use. Subjective effects last from 5 seconds to 15 minutes. Tolerance develops readily, but there are no known withdrawal symptoms. Abstinence for several days reestablishes the previous level of responsiveness. Long-term effects may include damage to the immune system and respiratory difficulties.
Sniffing of solvents and inhaling of gases (including aerosols) produce a form of inebriation similar to that of the volatile anesthetics. Agents include gasoline, toluene, petroleum ether, lighter fluids, cleaning fluids, paint thinners, and solvents that are present in many household products (eg, nail polish). Typical intoxication states include euphoria, slurred speech, hallucinations, and confusion, and with high doses, acute manifestations are unconsciousness and cardiorespiratory depression or failure; chronic exposure produces a variety of symptoms related to the liver, kidney, bone marrow, or heart. Lead encephalopathy can be associated with sniffing leaded gasoline. In addition, studies of workers chronically exposed to jet fuel showed significant increases in neurasthenic symptoms, including fatigue, anxiety, mood changes, memory difficulties, and somatic complaints. These same problems have been noted in long-term solvent abuse.
The so-called designer drugs are synthetic substitutes for commonly used recreational drugs. Common designer drugs include methyl analogues of fentanyl used as heroin substitutes. MDMA is also a designer drug with high abuse potential and purported neurotoxicity. Often not detected by standard toxicology screens, these substances can present a vexing problem for clinicians faced with symptoms from a totally unknown cause.
Manhapra A et al. Pain and addiction: an integrative therapeutic approach. Med Clin North Am. 2018 Jul;102(4):745–63. [PMID: 29933827]
NEUROCOGNITIVE DISORDERS
ESSENTIALS OF DIAGNOSIS

 Transient or permanent brain dysfunction with alterations in awareness or attention.
Transient or permanent brain dysfunction with alterations in awareness or attention.
 Cognitive impairment to varying degrees.
Cognitive impairment to varying degrees.
 Impaired recall and recent memory, inability to focus attention and problems in perceptual processing, often with psychotic ideation.
Impaired recall and recent memory, inability to focus attention and problems in perceptual processing, often with psychotic ideation.
 Random psychomotor activity such as stereotypy.
Random psychomotor activity such as stereotypy.
 Emotional disorders frequently present: depression, anxiety, irritability.
Emotional disorders frequently present: depression, anxiety, irritability.
 Behavioral disturbances: impulse control, sexual acting-out, attention deficits, aggression, and exhibitionism.
Behavioral disturbances: impulse control, sexual acting-out, attention deficits, aggression, and exhibitionism.
 General Considerations
General Considerations
The organic problem may be a primary brain disorder or a secondary manifestation of some general disorder. All of the cognitive disorders show some degree of impaired thinking depending on the site of involvement, the rate of onset and progression, and the duration of the underlying brain lesion. Emotional disturbances (eg, depression) are often present as significant comorbidities. The behavioral disturbances tend to be more common with chronicity, more directly related to the underlying personality or central nervous system vulnerability to drug side effects, and not necessarily correlated with cognitive dysfunction.
The causes of cognitive disorders are listed in Table 25–11.
Table 25–11. Etiology of delirium and other cognitive disorders (listed in alphabetical order).
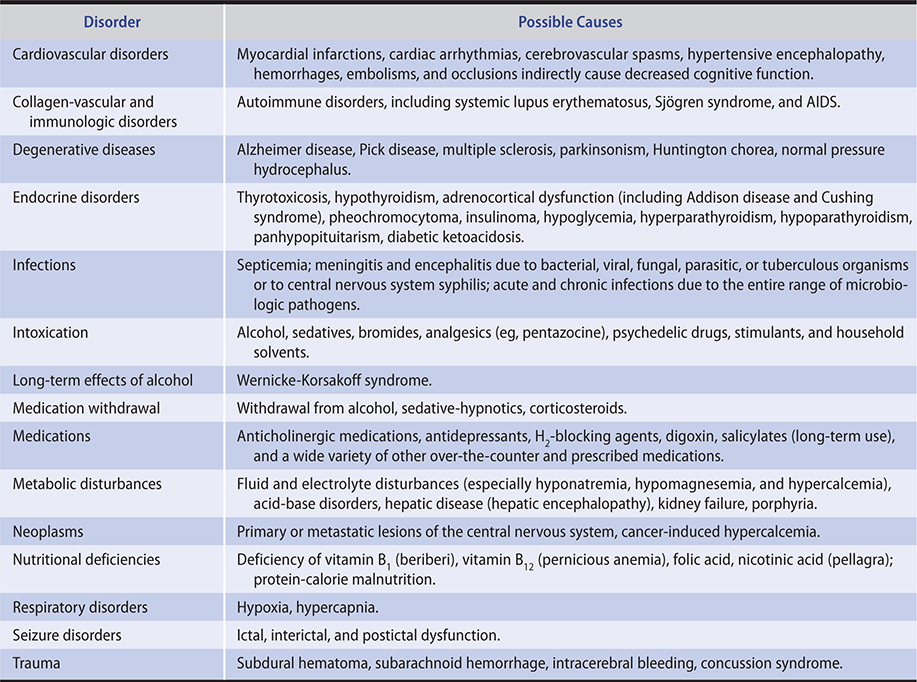
 Clinical Findings
Clinical Findings
The many manifestations include problems with orientation, short or fluctuating attention span, loss of recent memory and recall, impaired judgment, emotional lability, lack of initiative, impaired impulse control, inability to reason through problems, depression (worse in mild to moderate types), confabulation (not limited to alcohol organic brain syndrome), constriction of intellectual functions, visual and auditory hallucinations, and delusions. Physical findings will vary according to the cause. The electroencephalogram usually shows generalized slowing in delirium.
A. Delirium
Delirium (acute confusional state) is a transient global disorder of attention, with clouding of consciousness, usually a result of systemic problems (eg, medications, hypoxemia). See Chapters 4 and 24. Onset is usually rapid. The mental status fluctuates (impairment is usually least in the morning), with varying inability to concentrate, maintain attention, and sustain purposeful behavior. There is a marked deficit of short-term memory and recall. Anxiety and irritability are common. Orientation problems follow the inability to retain information. Perceptual disturbances (often visual hallucinations) and psychomotor restlessness with insomnia are common. “Sundowning”—mild to moderate delirium at night—is more common in patients with preexisting dementia and may be precipitated by hospitalization, medications, and sensory deprivation.
B. Dementia
Dementia is characterized by chronicity and deterioration of selective mental functions. See Chapters 4 and 24.
In all types of dementia, loss of impulse control (sexual and language) is common. Pseudodementia is a term previously applied to depressed patients who appear to be demented. These patients are often identifiable by their tendency to complain about memory problems vociferously rather than try to cover them up. They usually say they cannot complete cognitive tasks but with encouragement can often do so. They can be considered to have depression-induced reversible dementia that improves when the depression resolves. In many geriatric patients, however, the depression appears to be an insult that often unmasks a progressive dementia.
C. Amnestic Syndrome
This is a memory disturbance without delirium or dementia. It is usually associated with thiamine deficiency and chronic alcohol use (eg, Korsakoff syndrome). There is an impairment in the ability to learn new information or recall previously learned information.
D. Substance-Induced Hallucinosis
This condition is characterized by persistent or recurrent hallucinations (usually auditory) without the other symptoms usually found in delirium or dementia. Alcohol or hallucinogens are often the cause. There does not have to be any other mental disorder, and there may be complete spontaneous resolution.
 Treatment
Treatment
See Chapters 4 and 24 for detailed discussion.
PSYCHIATRIC PROBLEMS ASSOCIATED WITH HOSPITALIZATION & ILLNESS
 Diagnostic Categories
Diagnostic Categories
A. Acute Problems
1. Delirium with psychotic features secondary to the medical or surgical problem, or compounded by effect of treatment.
2. Acute anxiety, often related to ignorance and fear of the immediate problem as well as uncertainty about the future.
3. Anxiety as an intrinsic aspect of the medical problem (eg, hyperthyroidism).
4. Denial of illness, which may present during acute or intermediate phases of illness.
B. Intermediate Problems
1. Depression as a function of the illness or acceptance of the illness, often associated with realistic or fantasied hopelessness about the future.
2. Behavioral problems, often related to denial of illness and, in extreme cases, causing the patient to leave the hospital against medical advice.
C. Recuperative Problems
1. Decreasing cooperation as the patient sees that improvement and compliance are not compelled.
2. Readjustment problems with family, job, and society.
 General Considerations
General Considerations
A. Acute Problems
1. “Intensive care unit psychosis”—The ICU environment may contribute to the etiology of delirium. Critical care unit factors include sleep deprivation, increased arousal, mechanical ventilation, and social isolation. Other causes include those common to delirium and require vigorous investigation (see Delirium).
2. Presurgical and postsurgical anxiety states—Anxiety before or after surgery is common and commonly ignored. Presurgical anxiety is very common and is principally a fear of death (many surgical patients make out their wills). Patients may be fearful of anesthesia (improved by the preoperative anesthesia interview), the mysterious operating room, and the disease processes that might be uncovered by the surgeon. Such fears frequently cause people to delay examinations that might result in earlier surgery and a greater chance of cure.
The opposite of this is surgery proneness, the quest for surgery to escape from overwhelming life stresses. Some polysurgery patients may be classified as having factitious disorders. Dynamic motivations include the need to get medical care as a way of getting dependency needs met, the desire to outwit authority figures, unconscious guilt, or a masochistic need to suffer. Frequent surgery may also be related to a somatic symptom disorder, particularly body dysmorphic disorder (an obsession that a body part is disfigured). More apparent reasons may include an attempt to get relief from pain and a lifestyle that has become almost exclusively medically oriented, with all of the risks entailed in such an endeavor.
Postsurgical anxiety states are usually related to pain, procedures, and loss of body image. Acute pain problems are quite different from chronic pain disorders (see Chronic Pain Disorders, this chapter); the former are readily handled with adequate analgesic medication (see Chapter 5). Alterations in body image, as with amputations, ostomies, and mastectomies, often raise concerns about relationships with others.
3. Iatrogenic problems—These usually pertain to medications, complications of diagnostic and treatment procedures, and impersonal and unsympathetic staff behavior. Polypharmacy is often a factor. Patients with unsolved diagnostic problems are at higher risk. They are desirous of relief, and the quest engenders more diagnostic procedures with a higher incidence of complications. The upset patient and family may be very demanding. Excessive demands usually result from anxiety. Such behavior is best handled with calm and measured responses.
B. Intermediate Problems
1. Prolonged hospitalization—Prolonged hospitalization presents unique problems in certain hospital services, eg, burn units or orthopedic services. The acute problems of the severely burned patient are discussed in Chapter 37. The problems often are behavioral difficulties related to length of hospitalization and necessary procedures. For example, in burn units, pain is a major problem in addition to anxiety about procedures. Disputes with staff are common and often concern pain medication or ward privileges. Some patients regress to infantile behavior and dependency. Staff members must agree about their approach to the patient in order to ensure the smooth functioning of the unit.
Denial of illness may present in some patients. Intervention by an authority figure (eg, immediate work supervisor) may help the patient accept treatment and eventually abandon the coping mechanism of denial.
2. Depression—Mood disorders ranging from mild adjustment disorder to major depressive disorder frequently occur during prolonged hospitalizations. A key to the diagnosis of depression in the medical setting is the individual’s loss of self-esteem; they often think of themselves as worthless and are guilt ridden. Therapeutic medications (eg, corticosteroids) may be a factor. Depression can contribute to irritability and overt anger. Severe depression can lead to anorexia, which further complicates healing and metabolic balance. It is during this period that the issue of disfigurement arises—relief at survival gives way to concern about future function and appearance.
C. Recuperative Problems
1. Anxiety—Anxiety about return to the posthospital environment can cause regression to a dependent position. Complications increase, and staff forbearance again is tested. Anxiety occurring at this stage usually is handled more easily than previous behavior problems.
2. Posthospital adjustment—Adjustment difficulties after discharge are related to the severity of the deficits and the use of outpatient facilities (eg, physical therapy, rehabilitation programs, psychiatric outpatient treatment). Some patients may experience posttraumatic stress symptoms (eg, from traumatic injuries or even from necessary medical treatments). Lack of appropriate follow-up can contribute to depression in the patient, who may feel that he or she is making poor progress and may have thoughts of “giving up.” Reintegration into work, educational, and social endeavors may be slow.
 Clinical Findings
Clinical Findings
The symptoms that occur in these patients are similar to those discussed in previous sections of this chapter, eg, delirium, stress and adjustment disorders, anxiety, and depression. Behavior problems may include lack of cooperation, increased complaints, demands for medication, sexual approaches to nurses, threats to leave the hospital, and actual signing out against medical recommendations. The stress of hospitalization often brings out these more primitive defense mechanisms than the patient displays in daily life.
 Complications
Complications
Prolongation of hospitalization causes increased expense, deterioration of patient-staff relationships, and increased probabilities of iatrogenic and legal problems. The possibility of increasing posthospital treatment problems is enhanced.
 Treatment
Treatment
A. Medical
The most important consideration by far is to have one clinician in charge, a clinician whom the patient trusts and who is able to oversee multiple treatment approaches (see Somatic Symptom Disorders, above). In acute problems, attention must be paid to metabolic imbalance, alcohol withdrawal, and previous drug use—prescribed, recreational, or over-the-counter. Adequate sleep and analgesia are important in enhancing a patient’s coping abilities.
Many clinicians are attuned to the early detection of the surgery-prone patient. Plastic and orthopedic surgeons are at particular risk. Appropriate consultations may help detect some problems and mitigate future ones.
Postsurgical anxiety states can be alleviated by personal attention from the surgeon. Anxiety is not so effectively lessened by ancillary medical personnel, whom the patient perceives as lesser authorities, until after the clinician has reassured the patient. “Patient-controlled analgesia” can improve pain control, decrease anxiety, and minimize side effects.
Depression should be recognized early. If moderate to severe, antidepressant medications (see Antidepressant Medications, above) may be prescribed. High levels of anxiety can be lowered with judicious use of anxiolytic agents. Unnecessary medications tend to reinforce the patient’s impression that there must be a serious illness or medication would not be required.
B. Psychological
Prepare the patient and family for what is to come. This includes the types of units where the patient will be quartered, the procedures that will be performed, and any disfigurements that will result from surgery. Repetition improves understanding. The nursing staff can be helpful, since patients frequently confide a lack of understanding to a nurse but are reluctant to do so to the physician.
Denial of illness is frequently a block to acceptance of treatment. This too should be handled with family members present (to help the patient face the reality of the situation) in a series of short interviews (for reinforcement). Dependency problems resulting from long hospitalization are best handled by focusing on the changes to come as the patient makes the transition to the outside world. Key figures are teachers, vocational counselors, and physical therapists. Challenges should be realistic and practical and handled in small steps.
Depression is usually related to the loss of familiar hospital supports, and the outpatient therapists and counselors help to lessen the impact of the loss. Some of the impact can be alleviated by anticipating, with the patient and family, the signal features of the common depression to help prevent the patient from assuming a permanent sick role.
Suicide is always a concern when a patient is faced with despair. An honest, compassionate, and supportive approach will help sustain the patient during this trying period.
C. Behavioral
Prior desensitization can significantly allay anxiety about medical procedures. A “dry run” can be done to reinforce the oral description. Cooperation during acute problem periods can be enhanced by the use of appropriate reinforcers such as a favorite nurse or helpful family member. People who are positive reinforcers are even more helpful during the intermediate phases when the patient becomes resistant to the seemingly endless procedures (eg, debridement of burned areas).
Specific situations (eg, psychological dependency on the respirator) can be corrected by weaning with appropriate reinforcers (eg, watching a favorite movie on a DVD player or laptop when disconnected from the ventilator). Behavioral approaches should be used in a positive and optimistic way for maximal reinforcement.
Relaxation techniques, hypnosis, and attentional distraction can be used to block side effects of a necessary treatment (eg, nausea in cancer chemotherapy).
D. Social
A change in environment requires adaptation. Because of the illness, admission and hospitalization may be more easily handled than discharge. A predischarge evaluation must be made to determine whether the family will be able to cope with the physical or mental changes in the patient. Working with the family while the patient is in the acute stage may presage a successful transition later on.
Development of a new social life can be facilitated by various self-help organizations (eg, the stoma club). Sharing problems with others in similar circumstances eases the return to a social life, which may be quite different from that prior to the illness.
 Prognosis
Prognosis
The prognosis is good in all patients who have reversible medical and surgical conditions. It is guarded when there is serious functional loss that impairs vocational, educational, or societal possibilities—especially in the case of progressive and ultimately life-threatening illness.
Hshieh TT et al. Delirium in the elderly. Psychiatr Clin North Am. 2018 Mar;41(1):1–17. [PMID: 29412839]