Chapter 2
Basic Plan of the Nervous System
Introduction
The brain often is compared with a computer these days. True, the brain is a computer, but it is a very special kind of computer—a biological computer that has evolved by natural selection over hundreds of millions of years and countless generations. Furthermore, it has no obvious design features in common with human-engineered computers. Instead, the brain is a unique organ that thinks and feels, generates behavioral interactions with the environment, keeps bodily physiology relatively stable, and enables reproduction of the species—its most important role from evolution’s grand perspective. And for strictly personal reasons the brain is the most precious thing we have simply because it is the organ of consciousness, as reflected in René Descartes’s famous seventeenth-century aphorism, “I think, therefore I am.”
Aristotle first emphasized that structure and function are inextricably intertwined, two sides of the same coin, with structure providing obvious physical constraints on function. Just think about the difference between a hammer and a saw. Unfortunately, as knowledge becomes more and more specialized, there is a tendency to analyze the structure, function, and chemistry of the nervous system from different, sometimes even isolated, perspectives. The main theme of this chapter is the basic structure-function organization of the nervous system: what are the parts and how are they interconnected into functional systems? In other words, what are the organizing principles—the basic design features—of its circuitry?
Evolution and development are two approaches often used to understand biological complexity—because they start with the simplest condition—and the human brain is far and away the most complex object we know of, with its roughly 100 billion neurons and 100 trillion axonal connections between them. One remarkable conclusion emerging from these two perspectives is that nerve cells in all animals—from jellyfish to humans—are basically the same in terms of cell biology; what changes most during ontogeny and phylogeny is the arrangement of nerve cells into functional circuits: the architecture of the nervous system. The “bricks” or “Legos” are similar, but the “buildings” constructed with them can vary tremendously in size and functionality. An ultimate goal of neuroscience may be to understand the human brain, but remarkable progress can nevertheless be made through analyzing “lower” animals and early embryos. The other equally remarkable conclusion is that all vertebrates, from fish to humans, share a common basic plan of the nervous system, with the same major parts and functional systems.
Evolution Highlights: General Organizing Principles
Protists and the simplest multicellular animals (sponges) display ingestive, defensive, reproductive, and other behaviors without any nervous system whatsoever, raising the question, what is the adaptive value of adding a nervous system to an organism? We will now examine key structure-function correlates of nervous system organization in animals with relatively simple body plans and behaviors.
The Nerve Net is the Simplest Type of Nervous System
In his provocative 1919 book The Elementary Nervous System, George Parker outlined a reasonable scenario for how nervous systems evolved. An updated version begins with the first multicellular animals—similar to modern-day sponges—that emerged over half a billion years ago. They are seemingly amorphous animals that spend their adult lives immobile, submerged in water. Their relatively simple behavior is mediated largely by a set of primitive smooth muscle cell (myocyte) sphincters regulating water flow through body wall pores. These specialized cells are called independent effectors because their contraction is evoked by stimuli like stretch or environmental chemicals acting directly on the plasma membrane of individual cells.
The first animal phylum with a nervous system was the Cnidaria, which includes jellyfish, corals, sea anemones, and the elegantly simple hydra. In contrast to sponges, hydra locomote and show active feeding behavior (Fig. 2.1). These behaviors are coordinated and mediated by a nervous system, a network of specialized units or cells called nerve cells or neurons (Box 2.1).

Figure 2.1 Locomotor behavior in hydra resembles a series of somersaults, as shown in the sequence beginning on the left. The tiny black dot in the region between the tentacles in the figure at the far right is the animal’s mouth. Ingestive (feeding) behavior involves guiding food particles into the mouth with coordinated tentacle movements.
Box 2.1 The Neuron Doctrine
The cell theory, which states that all organisms are composed of individual cells, was developed around the middle of the nineteenth century by Mattias Schleiden and Theodor Schwann. However, this unitary vision of the cellular nature of life was not immediately applied to the nervous system, as most biologists at the time believed in the cytoplasmic continuity of nervous system cells. Later in the century, the most prominent advocate of this reticularist view was Camillo Golgi, who proposed that axons entering the spinal cord actually fuse with other axons (Fig. 2.2A). The reticularist view was challenged most thoroughly by Santiago Ramón y Cajal, a founder of contemporary neuroscience and without doubt the greatest observer of neuronal architecture. In beautifully written and carefully reasoned deductive arguments, Cajal presented us with what is now known as the neuron doctrine. This great concept in essence states that the cell theory applies to the nervous system: each neuron is an individual entity, the basic unit of neural circuitry (Fig. 2.2B). The acrimonious debate between reticularists and proponents of the neuron doctrine raged for decades. Over the years, the validity of the neuron doctrine has been supported by a wealth of accumulated data. Nevertheless, the reticularist view is not entirely incorrect, because some neurons do act syncytially via specialized intercellular gap junctions, a feature that is more prominent during embryogenesis.
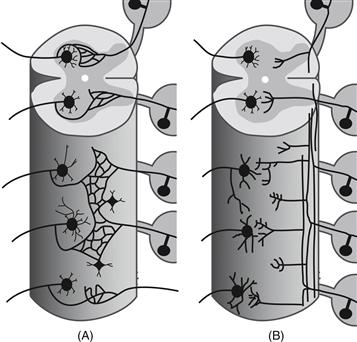
Figure 2.2 Two competing views: The nervous system as a reticulum or the neuron doctrine. (A) Proponents of the reticular theory believed that neurons are physically continuous with one another, forming an uninterrupted network. (B) In contrast, the neuron doctrine regards each neuron as an individual entity communicating with target cells by way of contiguity rather than continuity, across an appropriate intercellular gap.
Adapted from Cajal (1909–1911).
In 1897, Charles Sherrington postulated that neurons establish functional contact with one another and with other cell types via a theoretical structure he called the synapse (Greek synaptein, to fasten together). It was not until 50 years later that the structural existence of synapses was demonstrated by electron microscopy (see Fig. 3.3). The synaptic complex is built around an adhesive junction, and in this and other respects the complex is quite similar to the desmosome and the adherens junctions of epithelia. In fact, similarities in ultrastructure between the adherens junction and the synaptic complex of central nervous tissue were noted even in early electron microscopic studies (see Peters, Palay, & Webster, 1991).
Sensory Neurons
Hydra’s body wall is simple, with an outer ectoderm layer contacting the external environment, an inner endoderm layer facing the body cavity’s internal environment and promoting digestion and waste elimination, and a vague middle or meso layer in between. Neurons probably differentiated initially from the ectoderm, and perhaps the first to evolve were sensory neurons. One cytoplasmic extension of these bipolar cells facing the external environment became specialized to detect stimuli much weaker than those activating independent effectors, whereas the other pole became specialized to transmit information about these stimuli to a group of independent effectors (Fig. 2.3). Experimental evidence indicates that sensory neurons provide four major selective advantages in evolution:
• Increased stimulus sensitivity
• Faster effector cell responses
• Stronger behavioral responses because multiple effector cells are influenced
• Sensory neurons responding to different stimulus modalities can be distributed strategically in different body regions
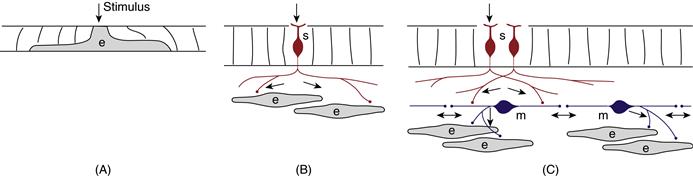
Figure 2.3 Activation of effector cells (e) in simple animals. (A) Sponges lack a nervous system; stimuli act directly on effector cells, which are thus called independent effectors. (B) In cniderians, bipolar sensory neurons (s) differentiate in the ectoderm. The sensory neuron outer extension detects stimuli and is thus a dendrite. The inner extension of some sensory neurons transmits information directly to effector cells and is thus an axon. Because this type of sensory neuron innervates effector cells directly, it is actually a sensorimotor neuron. (C) Most cniderian sensory neurons send their axon to motoneurons (m), which in turn send an axon to effector cells. Cniderian motoneurons may also have lateral extensions interacting with other motoneurons, and these extensions typically conduct information in either direction via reciprocal chemical synapses (and are thus amacrine extensions). Arrows show the direction of information flow.
The bipolar shape of sensory neurons is fundamentally important. The prototypical theory about neural circuit organization was presented by Santiago Ramón y Cajal in his classic “bible” of structural neuroscience, The Histology of the Nervous System in Man and Vertebrates (1909–1911). According to the cornerstone functional polarity theory, information normally flows in one direction through most neurons, and thus through most neural circuits—from dendrites and cell body, the input or receptive parts of the neuron, to a single axon, the output or effector part. In other words, most neurons have two classes of cytoplasmic extensions: one or more dendrites detecting inputs and a single axon conducting an output that can influence multiple cells through branching or collateralization. At least in early developmental stages, all sensory neurons have this fundamental bipolar shape, and over the course of evolution they have become specialized to detect a remarkable variety of stimuli from light, temperature, and a wide range of chemicals and ions, to vibration and other mechanical deformations.
Motor Neurons
A second stage of differentiation or complexity in hydra’s nervous system was the addition of neurons between sensory and effector. They are defined as motor neurons (motoneurons) because they directly innervate effector cells (usually muscle or gland cells), which in turn receive their inputs from sensory neurons (Figs. 2.2B, 2.3C). Conceptually, this provides a two-layered nervous system, with the first or top layer having sensory neurons, and the second or bottom layer having motor neurons. In this prototypical network sensory neurons project (send axon collaterals) to multiple motoneurons, and then each motoneuron innervates a set of effector cells (with a motoneuron and its effector cell set defined as a motor unit). During an animal’s normal behavior, information flow is unidirectional or polarized from one cell type, sensory neuron, to another cell type, motoneuron, to a third cell type, effector. This is the basic definition of a simple reflex, as defined by Charles Sherrington in his cornerstone of systems neuroscience, The Integrative Action of the Nervous System (1906).
In this hypothetical scenario (Fig. 2.3) an environmental stimulus detected by a sensory neuron’s dendrite is transmitted by its axon to the dendrites of a motoneuron population. Then the axon of each motoneuron innervates an effector cell population. This is the functional polarity rule applied to a simple two-layer, sensory-motor network mediating reflex behavior.
Another general feature of the hydra two-layered nervous system has been observed: sensory neurons do not innervate one another, whereas motoneurons do interact directly. Here, motoneurons have two projection classes: one to effector cells and another to other motoneurons. Structurally and functionally, many of these hydra motoneurons also have two types of output extensions. One is a typical axon innervating an effector cell population. However, the other is an extension that contacts homologous extensions from other motoneurons. Interestingly, many of these “tangential” extensions between motoneurons transmit information in either direction; either motoneuron can transmit information to the other via these extensions. This is an exception to the functional polarity rule and is mediated by reciprocal rather than the more common unidirectional synapses. Cajal (1909–1911) described several examples of neurons that lack a clear axon (in retina, olfactory bulb, and intestine) and called them amacrine cells. As an extension of this, it is useful to divide neuronal extensions into three types: dendritic (input), axonal (output), or amacrine (bidirectional).
Adding a second layer to the nervous system has obvious adaptive advantages related to increased capacity for response complexity and integration. Consider a stimulus to one specific part of the animal or even one sensory neuron. Its influence may radiate to distant parts of the animal because one sensory neuron innervates multiple motoneurons, those motoneurons innervate additional motoneurons, and each motoneuron innervates multiple effector cells—an example of what Cajal called “avalanche conduction.” There may be great divergence between stimulus and effector cells producing a response, with the actual divergence pattern shaped by the structure–function architecture of the nervous system: how the neurons and their interconnections are arranged in the body. It is easy to imagine how this arrangement in hydra might coordinate the tentacles to bring a food morsel detected by just one of them to the mouth or how it might coordinate locomotion (Fig. 2.1).
A second basic consequence of this structural arrangement is information convergence in the nervous system. Just consider a particular motoneuron: it can receive inputs from more than one sensory neuron and from other motoneurons as well.
Nerve Nets
At first glance, hydra’s nervous system is distributed fairly uniformly around the radially symmetrical body wall and tentacles (Fig. 2.4). Its essentially double-layered arrangement of distributed sensory and motor neurons is called a nerve net. However, in certain regions of the body with specialized function, like around the mouth and base of the tentacles, neurons tend to aggregate—a tendency toward centralization that will now be examined more carefully.
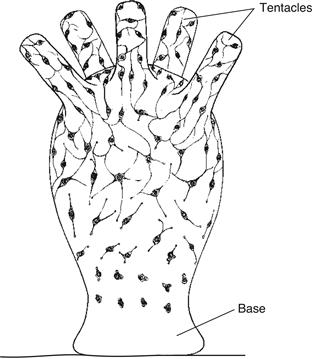
Figure 2.4 The nerve net of hydra, a simple cnidarian, is spread diffusely throughout the body wall of the animal. This drawing shows maturation of the nerve net in a hydra bud, starting near the base and finishing near the tentacles.
Refer to McConnell (1932) and Koizumi (2002).
Bilateral Symmetry, Centralization, and Cephalization Emerge in Flatworms
In contrast to cnidarians, flatworms are bilaterally symmetrical predators with rostral (head) and caudal (tail) ends, and dorsal and ventral surfaces. These changes in body plan and behavior are accompanied by equally important changes in nervous system organization. Many flatworm neurons are clustered into distinct ganglia interconnected by longitudinal and transverse axon bundles called nerve cords (Fig. 2.5). This condensation of neural elements, or centralization, allows faster and thus more efficient communication between neurons because cellular material is conserved and conduction times are reduced. The largest, most complex ganglia (cephalic ganglia or invertebrate brains) are localized rostrally, where they receive information from specialized sensory receptors in the front of the animal as it swims. Bilateral symmetry, centralization, and cephalization are three cardinal organizational trends in nervous system evolution.
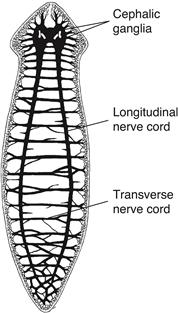
Figure 2.5 The nervous system of the planarian, a flatworm, includes longitudinal and transverse nerve cords associated with centralization, and two fused cephalic ganglia (the invertebrate brain) in the rostral end associated with cephalization. Centralization and cephalization probably are related to the flatworm’s bilateral symmetry and ability to swim forward rapidly.
Refer to Lentz (1968). Reproduced with permission from Yale University Press.
Interneurons
Flatworms are the simplest animals, with an abundant, clearly distinct third neuron division, interneurons, which are interpolated between sensory and motor neurons (Fig. 2.6). As already noted, Cajal recognized some atypical interneurons that apparently lack distinguishable dendrites and axon (amacrine neurons, or more precisely, amacrine interneurons). However, most interneurons have recognizable dendrites and axon and so presumably transmit information down the axon in only one direction: toward its terminals. They are typical neurons conforming to the functional polarity rule.
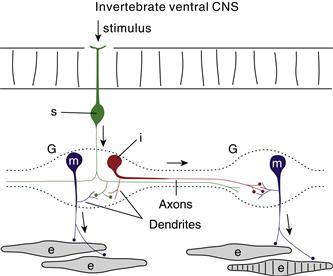
Figure 2.6 Invertebrate ganglia (G) usually display two neuron classes: motor neurons (m) and interneurons (i), both typically unipolar, with dendrites arising from a single axon. Here neuronal cell bodies are arranged peripherally and synapses occur in a central region called the neuropil. Sensory neurons (s) usually innervate motoneurons and interneurons but not effectors (e). Arrows show the usual direction of information flow.
One consequence of adding a third “layer” of neurons to the nervous system is simply to increase convergence and divergence of information processing, and thus the capacity for response complexity. There are, however, three other critical functions interneurons subserve. They can act as excitatory or inhibitory “switches” in neuronal networks, assemblies of them can act as pattern detectors and generators between sensory and motor neurons, and they can be pacemakers if they generate intrinsic rhythmical activity patterns.
By this definition the vast majority of vertebrate brain neurons are interneurons. So it is useful at the outset to recognize two broad interneuron categories: intraregional or local and interregional or projection. Intraregional interneurons, or local circuit neurons, have an axon that remains confined to a distinguishable gray matter region or ganglion, whereas interregional interneurons send a longer axon to a different gray matter region or ganglion, although it may also generate local axon collaterals.
Box 2.2 Cajal
Iconoclast To Icon
Santiago Ramón y Cajal (1852–1934) is considered by many people to be the founder of modern neuroscience—a peer of Darwin and Pasteur in nineteenth-century biology. He was born in the tiny Spanish village of Petilla de Aragon on May 1, 1852, and as related in his delightful autobiography, he was somewhat mischievous as a child and determined to become an artist, much to the consternation of his father, a respected local physician. However, he eventually entered the University of Zaragoza and received a medical degree in 1873. As a professor of anatomy at Zaragoza, his interests were mostly in bacteriology (the nineteenth-century equivalent of molecular biology today in terms of an exciting biomedical frontier) until 1887, when he visited Madrid at age 35 and first saw through the microscope histological sections of brain tissue treated with the Golgi method, which had been introduced in 1873. Although very few workers had used this technique, Cajal saw immediately that it offered great hope in solving the most vexing problem of nineteenth-century neuroscience: How do adult nerve cells interact with one another? This realization galvanized and directed the rest of his scientific life, which was extremely productive in terms of originality, scope, and accuracy.
Shortly after Jacob Schleiden, Theodor Schwann, and Rudolf Virchow proposed the cell theory in the late 1830s, Joseph von Gerlach Sr. and Otto Deiters suggested that nerve tissue was special in the sense that nerve cells are not independent units but instead form a continuous cytoplasmic syncytium or reticular net (Fig. 2.2A). This concept was later refined by Camillo Golgi, who, based on the use of his silver chromate method, concluded that axons of nerve cells form a continuous reticular net, whereas in contrast dendrites do not anastomose but instead serve a nutritive role, much like the roots of a tree. Using the same technique, Cajal almost immediately arrived at the opposite conclusion, based first on his examination of the cerebellum, and later of virtually all other parts of the nervous system. In short, he proposed that neurons interact by way of contact or contiguity rather than by continuity and are thus structurally independent units, which was finally proven when the electron microscope was used in the 1950s to characterize synapses. This concept became known as the neuron doctrine.
Cajal’s second major conceptual achievement was the theory of functional polarity, which stated that the dendrites and cell bodies of neurons receive information, whereas the single axon with its collaterals transmits information to the other cells. This rule allows prediction of information flow direction through neural circuits based on the morphology or shape of individual neurons forming them, and it was the cornerstone of Charles Sherrington’s (1906) revolutionary physiological analysis of mammalian reflex organization. Recent evidence that many dendrites transmit an action potential or graded potential in the retrograde direction would not violate the tenets of the functional polarity theory unless the potential led to altered membrane potentials in the associated presynaptic axon—and if this were the case the “dendrite” would be classed instead as an amacrine process (see text).
Around the close of the nineteenth century, Cajal made a remarkable series of discoveries at the cellular level. In addition to the two concepts outlined earlier, they include (1) the mode of axon termination in the adult CNS (1888), (2) the dendritic spine (1888), (3) the first diagrams of reflex pathways based on the neuron doctrine and functional polarity (1890), (4) the axonal growth cone (1890), (5) the chemotactic theory of synapse specificity (1892), and (6) the hypothesis that learning could be based on the selective strengthening of synapses (1895).
In one of the great ironies in the history of neuroscience, Cajal and Golgi shared the Nobel Prize for Medicine in 1906, though they had used the same technique to elaborate fundamentally different views on nervous system organization! The meeting in Stockholm may not have diminished the great personal friction between them. In 1931 Cajal wrote, “What a cruel irony of fate to pair like Siamese twins united by the shoulders, scientific adversaries of such contrasting characters.”
The omnidirectional information flow typical of cnidarian nerve nets is unusual in the rest of the animal kingdom, where most neurons are functionally polarized with information flowing through neural circuits sequentially from dendrites and cell body to axon and axon terminals. However, most invertebrate motoneurons and interneurons are unipolar: a single extension, the axon, emanates from the cell body. Dendrites branch from the axons in the center of a ganglion—entering the neuropil—where most synapses are formed (Fig. 2.6). In vertebrates most neurons are multipolar, with several dendrites, plus an axon extending from the cell body or a dendrite.
Features of simple nervous systems are preserved throughout evolution. For example, the part of the nervous system in the wall of the human gastrointestinal tract (the enteric plexuses) has many features of a highly refined nerve net, and a “layer” of amacrine interneurons is found in the human retina and olfactory bulb.
A Segmented Ventral Nerve Cord Typifies Annelids and Arthropods
Annelid worms and arthropods have even more complex body plans and behaviors than flatworms, partly because of segmentation. Body segments (metameres) are repeated serially along the body’s rostrocaudal axis and presumably share a common underlying genetic developmental program, although terminal differentiation (adult structure) may vary in each. This strategy allows for more complex body plans (including the nervous system) to evolve without a linear or exponential increase in genetic material. Annelids and all the more complex invertebrates share another characteristic feature, a ventral nerve cord with a pair of ganglia (or a single fused ganglion) in each segment, and longitudinal axon bundles between ganglia in adjacent segments (Fig. 2.7). Transverse nerves also extend from each ganglion to sensory structures and muscles in the same segment.
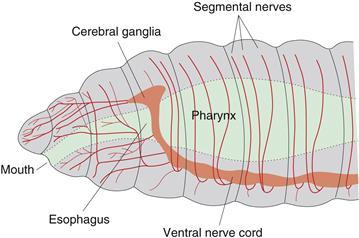
Figure 2.7 Nervous system organization in the rostral end of an annelid worm. A ventral nerve cord with more or less distinct ganglia connects with a fused pair of cerebral ganglia (brain) dorsal to the pharynx (part of the digestive tract). Note nerves extending from ventral nerve cord and cerebral ganglia.
Refer to Brusca and Brusca (1990).
The Basic Plan of the Vertebrate Nervous System is Found in Lancelets
Vertebrates are a subphylum of the Chordates and are the most complex of all animals in terms of structure and behavior. They share a basic body plan where common organ systems are arranged in a relatively strict anatomical relationship with one another (Box 2.3 and Figs. 2.8, 2.9). Like other Chordates, vertebrates display two key features during some part of their life: a cartilaginous rod, the notochord, extending dorsally along the body, and above it a hollow dorsal nerve cord. In most vertebrates the notochord’s body stiffening and protective functions are supplanted by the vertebral column and bony skull, with the notochord reduced to a series of cartilaginous cushions (discs) between or within the vertebrae. The vertebrate central nerve cord is tremendously expanded, thickened, and folded to form the brain and spinal cord (the central nervous system, CNS).
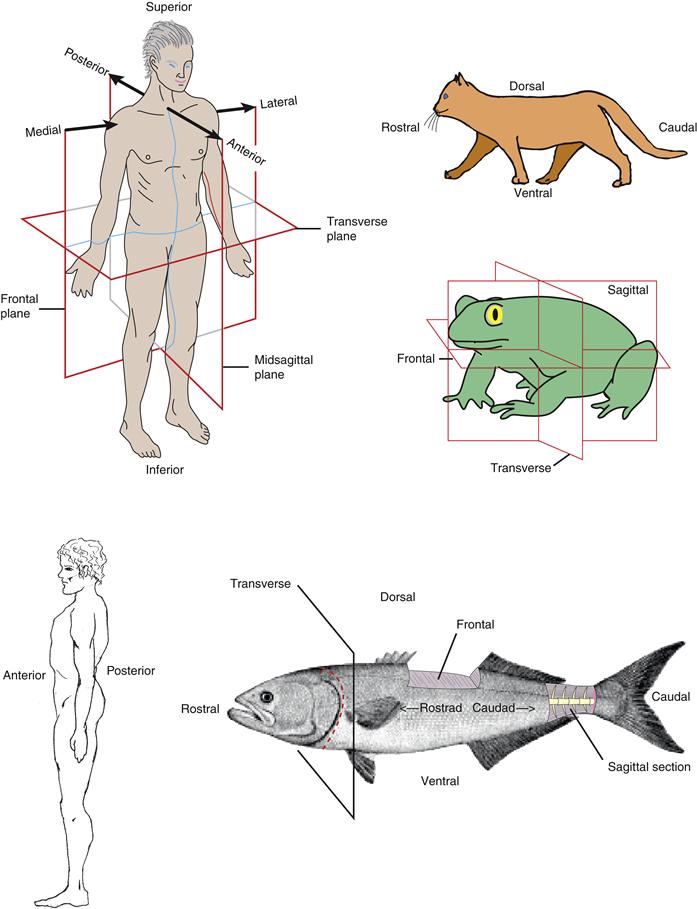
Figure 2.8 Orientation of the vertebrate body. Orientation planes for fish, quadrupeds, and bipeds are depicted. Associated with the three cardinal planes (rostrocaudal, dorsoventral, and mediolateral) are three orthogonal planes: frontal, sagittal, and transverse, which are the same in all early vertebrate embryos. For more explanation, see Williams (1995).
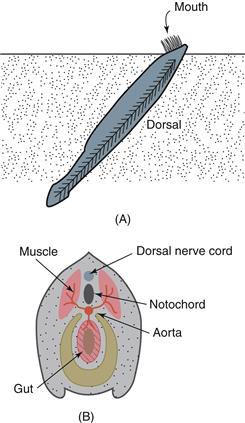
Figure 2.9 The lancelet (amphioxus) is a forerunner of the vertebrates. (A) Lateral view of the animal in its native habitat under the ocean floor, with its mouth protruding above the sand. (B) A cross-section of the lancelet body showing relationships between gut, aorta, notochord, and dorsal nerve cord.
Adapted from Cartmill, Hylander, and Shafland (1987).
The vertebrate nervous system’s basic parts are revealed in the lancelet (amphioxus), a simple, nonvertebrate chordate (subphylum Cephalochordata). The lancelet is a slender, fish-like filter-feeder living half buried in the sand of shallow, tropical marine waters (Fig. 2.9). The body is stiffened by a notochord, and a hollow dorsal nerve cord runs the length of the body, generating segmental nerves innervating muscles and organs. Locomotor behavior (swimming) is produced by alternately contracting right and left segmental muscles (myotomes). Without a notochord, these contractions would shorten the animal rather than generate forward propulsive force.
Although typical vertebrate brain regions are not obvious rostrally in the lancelet nerve cord, genes specifying early vertebrate head embryogenesis also are expressed rostrally in the lancelet body. Thus, some components of the molecular program specifying modern vertebrate head development apparently were present early in chordate evolution (Holland & Takahashi, 2005).
Box 2.3 Anatomical Relationships in the Vertebrate Body
To describe the physical relationships between structures in the nervous system and the rest of the vertebrate body, it is best to use terms that accurately and unambiguously describe the position of a given structure in three dimensions without reference to the external world. The major axis of the body is the rostrocaudal axis, which extends along the length of the animal from the rostrum (beak) to the cauda (tail) (Fig. 2.8), as well as the length of the embryonic neural plate and neural tube (see Figs. 2.10 and 2.12). A second axis, the orthogonal dorsoventral axis, is vertical and runs from the dorsum (back) to the ventrum (belly). Finally, the third perpendicular axis, the mediolateral axis, is horizontal and runs from the midline (medial) to the lateral margin of the animal (lateral). Unfortunately, the rostrocaudal axis undergoes complex bending during embryogenesis, and the bending pattern is unique to each species. It would be ideal if the three cardinal axes were used in a topologically accurate way—say, with reference to the body as it might appear with a “straightened out” rostrocaudal axis. In practice, however, this is rarely the case, which leads to a certain degree of ambiguity, as is obvious when looking at the fish, frog, cat, and human bodies shown in Figure 2.8.
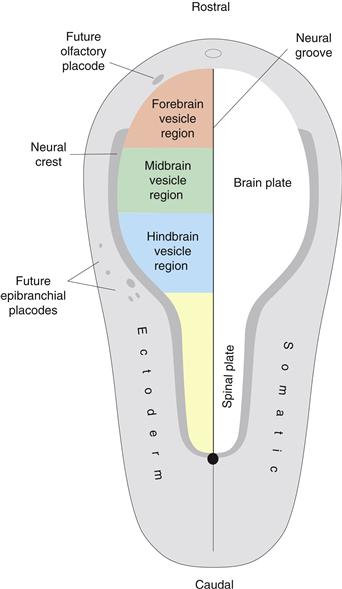
Figure 2.10 The neural plate is a spoon-shaped region of ectoderm (neural ectoderm) forming the CNS; surrounding it is somatic ectoderm. The neural plate is polarized (wider rostrally than caudally), bilaterally symmetrical (divided by the midline neural groove), and regionalized (brain plate rostrally, spinal plate caudally). The neural crest is a thin zone between neural and somatic ectoderm, and a series of placodes develops as “islands” within the somatic ectoderm. The neural crest and placodes generate PNS neurons. The approximate location of the future primary brain vesicles (Fig. 2.12A) in the neural plate is shown in color on the left. The same color scheme is used in Figs. 2.11, 2.12, and 2.14.
Refer to Swanson (1992).
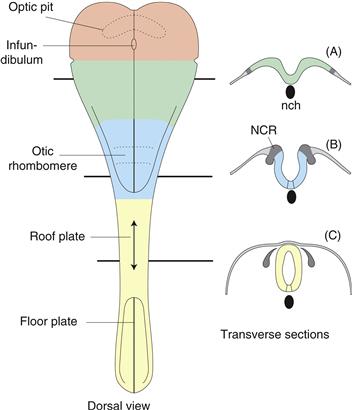
Figure 2.11 Optic pits, infundibulum, and otic rhombomere (dorsal view on left) are the earliest clear structural differentiations of the neural plate, other than the neural groove. The neural tube forms by neuroectoderm invagination (transverse sections A and B), followed by fusion of the lateral edges of the neural plate (roughly in the neck region of humans), and proceeds both rostrally and caudally (double arrows in roof plate). Note how the neural crest (NCR) pinches off in the process. Also observe notochord (nch) position ventral to neural groove.
Refer to Swanson (1992).
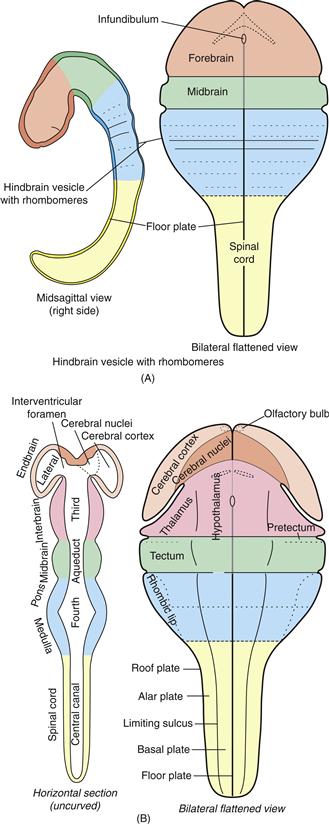
Figure 2.12 Formation and regionalization of the neural tube. (A) The early neural tube brain region develops three swellings: forebrain, midbrain, and hindbrain vesicles. The hindbrain vesicle then differentiates a series of transverse swellings called rhombomeres. (B) As differentiation continues, the forebrain vesicle displays right and left endbrain (cerebral hemisphere) vesicles and a medial interbrain vesicle, and the hindbrain vesicle shows vague pontine and medullary regions. This is the five-vesicle stage of neural tube transverse regionalization. Then longitudinal, dorsoventral, regionalization begins. The endbrain vesicle divides into cerebral cortex (including olfactory bulb) and cerebral nuclei (basal ganglia), the interbrain vesicle divides into thalamus and hypothalamus, the midbrain vesicle divides into tectum and tegmentum, the hindbrain vesicle becomes known as the rhombicbrain and divides into rhombic lip, alar plate, and basal plate, and the spinal cord divides into alar and basal plates. Whether the pretectal region (sometimes called synencephalon) is part of interbrain or midbrain is controversial. At this developmental stage major components of the adult ventricular system are seen in the neural tube lumen.
Refer to Swanson (1992) and Alvarez-Bolado and Swanson (1996).
The problem is especially difficult in human anatomy where use of an idiosyncratic terminology has a long, ingrained tradition. The basic principles are much easier to illustrate than to describe in writing (see Fig. 2.8), but one major source of confusion in the human brain is related to the fact that the rostrocaudal axis makes a 90-degree bend in the midbrain region (unlike in rodents and carnivores, for example, where the axis is relatively straight). The other source of confusion is simply the different names that are used. For example, in human anatomy the spinal cord has anterior and posterior horns, and posterior root ganglia, whereas in other mammals they usually are referred to as ventral and dorsal horns, and dorsal root ganglia. The merits of a uniformly applied nomenclature based on comparative structural principles seem obvious.
Summary
The cniderian nerve net displays most of the basic cellular features of nervous system organization, including convergence and divergence of sensory and motor information. In more complex bilaterally symmetrical invertebrates, neurons and axons tend to aggregate in ganglia, nerve cords, and nerves (centralization), and there is a greater concentration of neurons and sensory organs in the body’s rostral end (cephalization). Segmented invertebrates have a ventral nerve cord that includes a bilateral pair of ganglia (or single fused ganglion) in each segment. The primitive chordate, lancelet, displays the basic nervous system organization characteristic of vertebrates, including mammals and humans.
Development Reveals Basic Vertebrate Parts
One nineteenth-century biology triumph was the demonstration that early stages of embryogenesis are fundamentally the same in all vertebrates. The CNS and heart are the first organs to differentiate in the embryo, and the basic CNS divisions differentiating early in development are also common to all vertebrates. The names and arrangement of these divisions are the starting point for regional or topographic neuroanatomical nomenclature (Swanson, 2000a).
Nervous System Regionalization Begins in the Neural Plate
During embryogenesis the CNS develops as a hollow cylinder (neural tube) from a topologically flat sheet of cells (neural plate), by a process of neurulation (Chapter 13). Here we simply consider macroscopic structural changes during the transformation.
The neural plate is a spoon-shaped differentiation of the trilaminar embryonic disc’s one-cell-thick ectodermal layer (Fig. 2.10). Its wide end lies rostrally and becomes the brain, whereas the narrow end lies caudally and becomes the spinal cord—the two major CNS divisions. A midline neural groove divides the neural plate into right and left halves, so the plate displays three cardinal morphogenetic features: polarity, bilateral symmetry, and regionalization. Furthermore, the neural plate differentiates from rostral to caudal, so the brain plate regionalizes first. Signs of this include appearance of the optic vesicles, evaginating near the rostral end of the neural plate (in the presumptive hypothalamus); a midline infundibulum evaginating between optic vesicles and rostral end of the notochord, and indicating the presumptive pituitary stalk (again in presumptive hypothalamus); and the otic rhombomere (presumptive or future rhombomere 4), a swelling near the center of what will become the adult rhombicbrain (Figs. 2.11, left; 2.14).
At the junction between neural plate and remaining ectoderm (later forming the skin’s epidermal layer or skin) lies a narrow strip of transitional ectoderm, the neural crest, a distinctive vertebrate feature (Fig. 2.10). It generates a variety of adult structures, including most neurons of the peripheral nervous system (PNS).
In summary, the CNS and PNS divisions are represented by the neural plate and neural crest, respectively, during the neural plate stage of vertebrate development. The two major CNS divisions, the brain and spinal cord, are also indicated in the neural plate, which at this developmental stage is topologically simple: a bilaterally symmetrical, flat sheet that is one cell thick.
Further Regionalization Occurs in the Neural Tube
As neurulation progresses, the neural plate becomes U-shaped as the two halves (neural folds) become vertically oriented (Fig. 2.11, right). Then the dorsal tips of the folds fuse, forming an open tube—and finally the tube’s ends (neuropores) also fuse, producing a completely closed neural tube with a one-cell-thick wall (neuroepithelium).
Marcello Malpighi, the great seventeenth-century founder of histology, who also discovered the capillary network between arteries and veins postulated by William Harvey in 1628, recognized that the early chick neural tube displays three rostrocaudally arranged swellings now called primary brain vesicles. They include the forebrain (prosencephalic) vesicle, with the optic stalks and infundibulum; the midbrain (mesencephalic) vesicle; and the hindbrain (rhombencephalic) vesicle, with the otic rhombomere (Fig. 2.12A).
These vesicles are the fundamental structural or regional brain divisions. Transitory rhombomeres are the most characteristic primary hindbrain vesicle feature at this stage, and they develop in association with the pharyngeal pouches (Chapter 13). As embryogenesis continues, the forebrain vesicle divides into endbrain (telencephalic) and interbrain (diencephalic) vesicles, whereas the hindbrain vesicle becomes the rhombicbrain, which differentiates vaguely into two regions: rostrally the (secondary) hindbrain (epencephalon) and caudally the medulla (metencephalon); see Figure 2.12B. These divisions transform the “three primary vesicle stage” into the “five secondary vesicle stage,” with the hindbrain then producing a dorsal cerebellum and a ventral pons.
The neural tube lumen becomes the adult CNS ventricular system (Fig. 2.12B, left), and its adult shape conforms to extensive differential regionalization of the neural tube wall. Each endbrain vesicle contains a lateral ventricle, which communicates through an interventricular foramen with the third ventricle in the interbrain vesicle’s center. The third ventricle continues into the midbrain’s cerebral aqueduct, which becomes the rhombicbrain’s fourth ventricle and then the spinal cord’s central canal. In older embryos and adults, the ventricular system contains cerebrospinal fluid (CSF), much of which is elaborated by specialized, highly vascular regions of choroid plexus in the roof of the lateral, third, and fourth ventricles.
Migrating Neurons form the Mantle Layer’s Gray Matter
In the early five secondary vesicle neural tube, cells divide repeatedly, although the neural tube remains one cell thick, a pseudostratified epithelium of stem cells for neurons and glia. Shortly thereafter many of these cells begin a terminal differentiation into young neurons migrating from the luminal proliferation zone to form a new, more superficial mantle layer (Chapter 16). In some CNS regions, mantle layer neurons segregate into layers parallel to the surface, whereas in other regions, neurons cluster in nuclei, relatively uniform neuron populations (usually multiple types) that are structurally distinct from surrounding nuclear or layered regions.
Mantle layer formation leads to further CNS regionalization (Fig. 2.12B). In rhombicbrain and spinal cord, it emerges because motoneurons are generated earliest and ventrally (corresponding to medial neural plate regions). This correlates with observations that gross, relatively uncoordinated embryonic motor behavior starts before reflex pathways become functional—and implying that such behavior is generated endogenously in the CNS itself (Hamburger, 1973).
Ventral mantle layer formation accompanies the transient appearance of a longitudinal groove (limiting sulcus) on the neural tube’s inner surface. The leading nineteenth-century Swiss embryologist Wilhelm His noted that the limiting sulcus divides much of the neural tube into dorsal or alar plate and ventral or basal plate, with predominantly sensory and motor functions, respectively (Fig. 2.13). This observation complemented the earlier fundamental discovery of François Magendie that spinal sensory and motor fibers are completely segregated in spinal roots: sensory axons enter through dorsal roots, whereas motor axons leave through ventral roots. It is now clear that alar and basal plates are not purely sensory or motor because each contains projection interneurons. Nevertheless, it is helpful to view the rhombicbrain and spinal cord as having three longitudinal zones: sensory, integrative (reticular formation), and motor. Regionalization of midbrain and forebrain does not fit this scheme neatly and is relatively poorly understood conceptually.
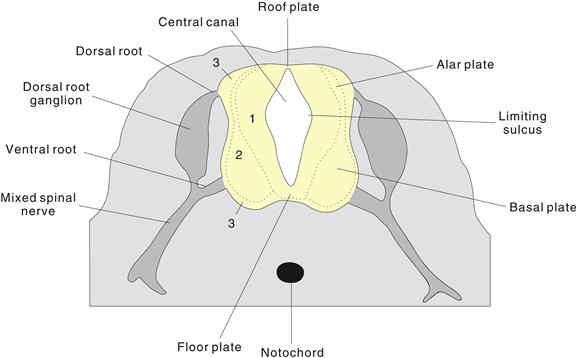
Figure 2.13 The early spinal cord and rhombicbrain are divided into dorsal (alar) and ventral (basal) plates by the limiting sulcus. This morphology reflects earlier ventral differentiation of the mantle layer (2), accompanied by earlier ventral thinning of the neuroepithelial or ventricular layer (1) of the neural tube, which remains as the adult ependymal lining of the ventricular system. The mantle layer develops into adult gray matter. This schematic drawing of a transverse spinal cord histological section also shows dorsal (sensory) and ventral (motor) spinal cord roots, dorsal root ganglia containing sensory neurons derived from the neural crest, and mixed (sensory and motor) spinal nerves distal to the ganglia. The peripheral area (3) is called the marginal zone and develops into the spinal cord white matter or funiculi containing ascending and descending axonal fiber tracts.
Dorsal regions of the rhombicbrain alar plate form a unique structure, the rhombic lip. In the rostral half it generates cerebellar granule cells, whereas more caudally it produces neuron populations like the precerebellar and vestibulocochlear nuclei. Many rhombic lip neuron populations are interesting because they migrate tangentially, parallel to the neural tube’s surface to reach their final destinations, instead of radially (transversely) like most CNS neurons (Chapter 16). This differentiation continues until the adult CNS configuration is achieved (Figs. 2.14 and 2.15). The most obvious late-developing structures are the cerebral cortex and cerebellar cortex.
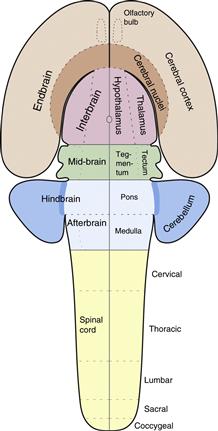
Figure 2.14 Major divisions of the adult mammalian CNS are derived from neural plate and neural tube regionalization illustrated in Figs. 2.10–2.12.
Modified from Swanson (1992).
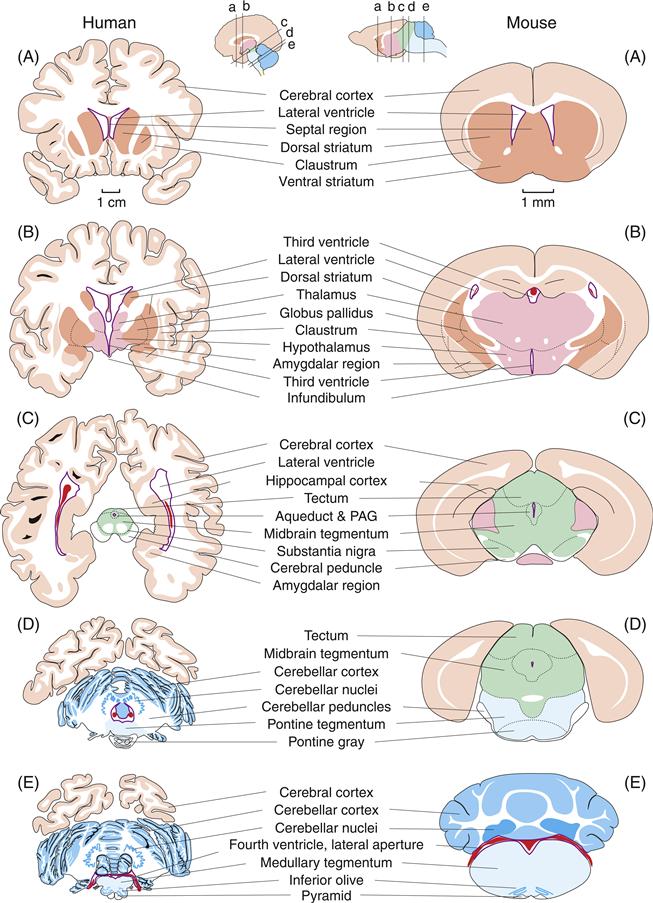
Figure 2.15 Mini atlases to compare major adult brain regions in humans and mice. The brains are cut approximately transversely to the CNS longitudinal axis and illustrate five major levels, arranged from rostral to caudal: a, endbrain; b, interbrain; c, midbrain; d, pons; and e, medulla. The color scheme follows that in Figs. 2.10–2.12 and 2.14, with the choroid plexus of the lateral, third, and fourth ventricles shown in red.
Adapted from Nieuwenhuys, Voogd, and Van Huijzen (1988) and Sidman, Angevine, and Webster (1971).
Summary
The vertebrate CNS develops from a sheet of cells called the neural plate that invaginates to form the neural tube. The tube’s rostral end differentiates a series of vesicles that constitute the major brain regions, and the caudal end forms the simpler spinal cord. Most PNS neurons differentiate from the neural crest, with the rest arising from nearby somatic ectodermal placodes.
The Basic Plan of Nervous System Connectivity
Functional Systems Consist of Interconnected Gray Matter Regions
The nervous system’s wiring diagram can be described in terms of the neuron types in each of its distinct gray matter regions and their stereotyped pattern of axonal connections to cell types both locally (within the region) and in other gray matter regions or other tissues (like muscle or gland). A long-term goal of systems neuroscience is to provide a global wiring diagram for the nervous system that systematically accounts for its various functional subsystems—analogous to the circulatory system model provided by Harvey. Little work has been done on this synthetic problem, although interest is accelerating with the development of online neuroinformatics workbenches for connectional information (Bota & Swanson, 2010) and with plans for complete tables of nervous system connections—connectomes (Sporns, Tonono, & Kötter, 2005).
The high-level model of nervous system information processing shown in Figure 2.16 synthesizes basic neurobiological concepts pioneered by Cajal and Sherrington with basic cybernetic principles pioneered by Norbert Wiener (1948) and John von Neumann (1958). In essence, the model postulates that behavior is determined by CNS motor system output and that this output is a function of three inputs: sensory system (reflexive), cognitive system (voluntary), and intrinsic behavioral state system. The relative importance of each input in controlling motor output (behavior) varies qualitatively in different species and quantitatively in different individuals. Note that behavior elicits sensory feedback from the external and internal environments that helps determine future motor activity and thus behavior. Each component is now considered further without trying to place all nervous system parts within the global model.
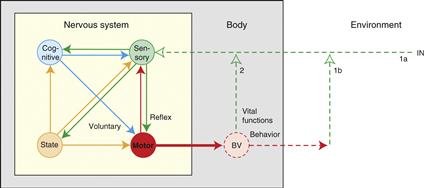
Figure 2.16 A model of the nervous system’s basic wiring diagram. The model of information flow through the nervous system (yellow box) postulates that behavior and vital body functions are controlled by the motor system (red), which is influenced by three classes of neural input: sensory (green), intrinsic behavioral state (gold), and cognitive (blue). Sensory inputs lead directly to reflex responses, cognitive inputs mediate voluntary responses, and intrinsic inputs act as control signals to regulate behavioral state. Motor system outputs produce behaviors whose consequences are monitored by sensory feedback (1b, 2). Sensory feedback may be used by the cognitive system for perception and by the intrinsic system to generate affect (e.g., positive and negative reinforcement/pleasure and pain). The cognitive, sensory, and intrinsic systems are all strongly interconnected in the indicated pattern.
Refer to Swanson (2012).
Motor Systems are Organized Hierarchically
There are three different motor systems: skeletal, autonomic, and neuroendocrine. The first controls striated muscles responsible for voluntary behavior; the second controls smooth and cardiac muscle, and many glands; and the third controls pituitary gland hormone secretion. The skeletal motor system is understood best and thus serves as a prototype for examining basic organizing principles presumably similar for all three.
The skeletal motor system is arranged hierarchically (Fig. 2.17), the lowest level consisting of brainstem-spinal cord α-motoneurons whose axons synapse directly on striated muscle fibers. The next higher level consists of motor pattern generators (MPGs), and the highest level has motor pattern initiators (MPIs) that “recognize” or alter their output in response to specific input patterns and project to unique sets of MPGs. Ethologists refer to MPIs as “innate releasing mechanisms.” One reason central neural circuitry is so complex is that each of the three input types (sensory, intrinsic, cognitive) may go directly to each general level of the motor system hierarchy.
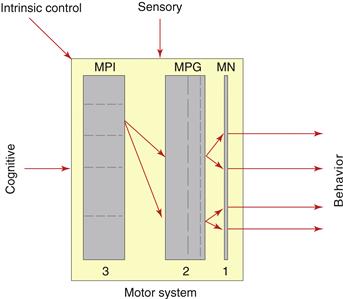
Figure 2.17 Hierarchical organization of the skeletal motor system. At the simplest level (1), motoneuron pools (MN) innervate individual muscles generating individual components of behavior. At the next higher level (2), additional interconnected interneuron pools, called motor pattern generators (MPG), innervate specific motoneuron pool sets. At the highest level (3), additional interconnected interneuron pools, called motor pattern initiators (MPI), innervate specific MPG sets. MPIs can activate complex, stereotyped behaviors when activated (or inhibited) by specific patterns of sensory, intrinsic, and/or cognitive inputs. Note that MPGs and MPIs themselves may be organized hierarchically (dashed lines) and that sensory, intrinsic, and cognitive inputs may go directly to any level of the motor system hierarchy.
Refer to Swanson (2012).
The MPGs and MPIs themselves are hierarchically arranged. This organization is particularly easy to see conceptually for the MPGs subserving locomotor behavior. In the spinal cord, simple MPGs coordinate the reciprocal innervation of muscle pair antagonists across individual joints, more complex MPGs coordinate activity in the set of simpler MPGs for all the joints in a limb, and still more complex MPGs coordinate activity in MPGs for all four limbs. At the next higher level there is a brain hierarchy of MPIs for locomotion that is activated by specific input patterns and projects to the spinal locomotor pattern generator network.
Multiple Sensory Systems Function in Parallel
A set of sensory systems provides information to the CNS from various receptor types, and all the systems can function simultaneously. Cajal noted that unimodal sensory pathways generally branch with some information going directly to the motor system and some going to the cerebral cortex for sensation and perception. The former typically evokes reflex behavior, and the latter potentially reaches consciousness and plays an important role in cognition.
Several general features characterize the sensory system (Section IV covers subsystems in detail). First, the CNS receives a wide range of information about the external environment and about the body’s internal state. Thus, sensory receptors lie near the body’s surface (e.g., touch and olfactory receptors), deep within the body (e.g., aortic stretch receptors), and even within the brain itself (e.g., hypothalamic insulin receptors). Second, each of the three motor systems receives a broad range of sensory inputs. Third, the range of sensory modalities is remarkably similar (though not identical) across vertebrate classes, and information about specific modalities enters the CNS through homologous cranial and spinal nerves in all vertebrates. And fourth, the number of synapses between sensory receptor and cerebral cortex varies in different systems. There is one synapse in the olfactory system and at least four in the visual system.
The Cognitive System Generates Anticipatory Behavior
It is very likely that the cerebral cortex—along with its cerebral nuclei (basal ganglia)—is the most important, if not sole, part of the cognitive system and that the cerebral cortex is responsible for planning, prioritizing, initiating, and evaluating the consequences of voluntary behavior (Section VII). The fundamental nature of voluntary behavior is obviously a difficult problem to address, but one useful approach is simply to compare it with reflexive behavior. Interestingly, most if not all behaviors mediated by skeletal muscle can be initiated either reflexively or voluntarily, as Descartes pointed out long ago. What seems to distinguish reflexive and voluntary behaviors most clearly is that the former involves a stereotyped response to a defined stimulus, whereas the latter is anticipatory, with a duration and content impossible to predict with anywhere near the same degree of certainty.
Intrinsic Systems Control Behavioral State
The CNS generates considerable endogenous activity (action potential patterns); it is definitely not just a passive system waiting to respond to sensory input, as the behaviorist approach a century ago assumed. All CNS parts apparently have a basal activity level that can be either increased or decreased. In many cases, it is still not established whether particular neuron types generate intrinsic activity patterns. It is clear, however, that motoneurons and related MPGs do generate intrinsic activity; as already noted, the embryonic spinal cord produces motor output before sensory circuits develop. Thus, in addition to the three extrinsic input types to the motor system illustrated in Figure 2.16, intrinsic activity within the motor system itself can produce behavior that is neither reflexive nor voluntary.
Certain CNS regions generate intrinsic rhythmic activity patterns. The most important rhythmic behavioral pattern is the sleep–wake cycle that is entrained to the day-night cycle by an endogenous circadian clock, the hypothalamic suprachiasmatic nucleus (Chapters 39 and 40). The sleep–wake cycle is profoundly important because during sleep the body is maintained entirely by ongoing intrinsic and reflexive systems controlling behaviors like respiration and sustained sphincter contractions. In contrast, voluntary mechanisms dominate in wakefulness though reflexive and intrinsic mechanisms are also vitally important then.
Behavioral state control is thus a fundamental intrinsic brain activity. Another aspect of behavioral state—arousal—is especially important during wakefulness. Arousal level generally is correlated with an animal’s motivational state or drive level (Chapter 41). The neural system mediating drive is not fully elucidated but is critically dependent on the hypothalamus, and attainment of specific goal objects (foraging behavior) depends on the cognitive system. Arousal and drive may be controlled by subcortical systems but behavior’s actual direction and prioritization mainly is determined cortically.
The full identity of neural systems elaborating pleasure and pain is one of neuroscience’s deep mysteries. Many regard pleasure and pain as conscious expressions of positive and negative reinforcement, influencing how likely a particular voluntary behavior will be repeated or avoided in the future. Here, reinforcement depends on sensory feedback about a particular behavior’s consequences (Fig. 2.16), and one suggestion is that pleasurable and painful sensations, like those associated with drive, are elaborated subcortically within intrinsic control systems. According to this view, thinking or cognition arises in cerebral cortex, whereas feeling or affect arises subcortically. It is also possible that all aspects of consciousness (thinking and feeling) arise only from cortical neural activity (Chapter 51).
How Pharmacological and Genetic Networks Relate to Functional Systems
Specific neurotransmitter systems have been incorporated into models of CNS function since the 1950s. Two examples are cholinergic and noradrenergic systems, defined as the total sets of CNS neurons releasing acetylcholine or noradrenalin, respectively, as a neurotransmitter (Chapter 5). In general, these systems are not obviously correlated with traditional CNS functional systems or major topographic parts; typically they are not restricted to one functional system or one major CNS division, though some exceptions may exist. Thus, neurotransmitter systems are not functional systems in the traditional sense. However, they are conceptually or operationally important in helping define networks or functional systems influenced by particular drug actions. For example, administering centrally acting acetylcholine receptor agonists influences synapses in a variety of traditional functional systems, and the set of these functional systems could be defined as a pharmacological system with a specific set of behavioral and other responses. If a drug is targeted for therapeutic reasons to a specific neural system (e.g., a cholinergic agonist targeted to the cerebral cholinergic system in Alzheimer’s disease; Chapter 43), it will also act on other functional systems with appropriate cholinergic receptors (e.g., in the thalamus and lower brainstem). Responses in these other systems produce “side effects” that may be good or bad.
Likewise, any gene product’s distribution pattern can also be used to define a chemical, molecular, or neural gene expression system. For example, a system could be defined in terms of all neurons expressing the calbindin or μ-opioid receptor gene, and expression of the corresponding gene might be prevented or altered in experimental knockout mice or natural mutations in genetic diseases. These alterations may produce an obvious and stereotyped phenotype or syndrome, but in most instances the gene normally is expressed in multiple functional systems and has complex (even if subtle) physiological and behavioral effects.
Finally, it is important to remember that a genetic program constructs the nervous system’s basic macrocircuitry during embryogenesis. Determining the correspondence between gene expression networks and neural networks may be the ultimate achievement of systems neuroscience. The nervous system’s microcircuitry—quantitative aspects of synapse number and strength associated with individual neurons—may be sculpted by experience throughout life.
Summary
There is no simple relationship between the CNS’s topographic or regional differentiation and its functional organization. So it is mistaken to assume a priori that CNS information simply is processed hierarchically with the spinal cord at the lowest level and the cerebral cortex at the top. An alternative view is that the CNS displays a network rather than hierarchical organization scheme—a circuit where the motor system is driven by sensory, cognitive, and intrinsic behavioral state inputs, and future motor activity is determined partly by sensory feedback about the initial behavior’s consequences.
Two major features complicate this simple network model. First, the motor system itself is organized hierarchically, whereas the sensory system transmits multiple modalities in parallel, and this sensory information can reach directly each level of the motor system hierarchy. And second, sensory information also reaches the intrinsic and cognitive systems. In fact, all three input systems are interconnected in a genetically determined pattern. The basic plan of neural circuit architecture must be understood on its own terms, not through simple preconceived ideas or superficial analogies with computers, the Internet, irrigation systems, or complicated robots. How traditional CNS functional systems relate to pharmacological systems and genetic networks remains to be determined.
Overview of the Adult Mammalian Nervous System
This section reviews structural neuroscience methods used to achieve our current—still very incomplete—understanding of nervous system architectural principles and introduces the major nervous system components. Long experience teaches that nothing approaches actual dissection for gaining an appreciation of overall brain structure.
A Brief History of Structural Neuroscience Methods
The human brain’s macroscopic structure was observed by early Greek physician-philosophers and thoroughly understood by the early 1800s. However, its circuitry’s astounding complexity was not appreciated until microscopy effectively identified individual pathways (axon bundles) and neuronal regions (distinguishable neuronal cell body aggregates) toward the end of the 1800s—applying to thin CNS tissue sections neurohistological reagents and reactions developed by the textile and photographic industries (Swanson, 2000b).
Perhaps the single most enduring contribution of nineteenth-century neurohistology was Camillo Golgi’s 1873 silver impregnation method. The full morphology of individual neurons was visible for the first time (Fig. 2.18; Boxes 2.1 and 2.2)—their dendritic tree, cell body shape, axon and all its collaterals, and points of presumed functional contact with other cells, which Sherrington named “synapses” in 1897. The Golgi method involves impregnating brain tissue alternately in potassium dichromate and silver nitrate solutions over periods of weeks to years, and mysteriously (the reaction’s chemistry remains elusive) about 1% of the neurons are filled (apparently randomly) with a dense precipitate. The Golgi stain thus reveals more by staining less. Today, selective labeling of individual neurons also is achieved by injecting markers directly into living neurons with micropipettes, allowing simultaneous electrophysiological and cytoplasm analysis.
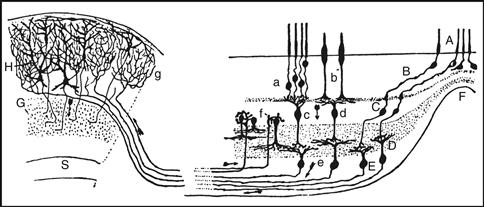
Figure 2.18 Cajal’s (1909–1911) neural architecture drawing based on the Golgi method. It shows the organization of four major retinal neuron types (right) and connections between two gray matter regions: retina to optic tectum (superior colliculus; left). Applying the neuron doctrine and functional polarity rule (Boxes 2.1 and 2.2) to the entire vertebrate nervous system by Cajal and many other researchers a century ago led to the “classical” way neuron types defining each gray matter region have been described structurally ever since, a view beautifully illustrated here. Three major neuron types tend to populate specific retinal layers: photoreceptors (subtypes a, b, A, B), bipolar cells (subtypes c, d), and ganglion cells (subtypes e, D, E). A specific neuron type’s cardinal feature is its axon’s distribution—the neuron’s function in terms of output. Photoreceptors detect light and their axon innervates bipolar cells. The latter in turn innervate ganglion cells whose axon projects through the optic nerve to the tectum. Photoreceptors are classical sensory neurons (Fig. 2.3), bipolar cells are local interneurons, and ganglion cells are projection interneurons. Also note a second retinal local interneuron class, amacrine cells (f). Cajal pointed out that retinal neuronal cell bodies aggregate in three layers with synaptic neuropil zones in between, and illustrated a clear structural gradient—reflecting a foveal region (F) with greater visual acuity because of multiple structural features, some of which are obvious in the drawing. The power of Cajal’s functional polarity theory is evident: he drew arrows to indicate the presumed normal direction of information flow through the network, based on the sequential arrangement of dendrites and axons associated with each neuron type.
Unfortunately, Golgi’s method provided little information about longer CNS connections: axonal projections between nonadjacent regions. This was approached with methods selectively staining fibers degenerating from pathological or experimental lesions. Augustus Waller showed (1850) that nerve transection causes the nerve’s distal segment to degenerate (Wallerian, anterograde degeneration), inspiring his proposal that the cell body is the nerve cell’s “trophic center,” which the axon depends on for survival. Thirty years later Bernard von Gudden showed that Wallerian degeneration may be accompanied by pathological changes in the cell body when its axon is cut, suggesting retrograde transport of “trophic factors” from axon to cell body.
Marchi and Algeri developed the first method to stain selectively central pathways (1885), revealing degenerating myelin sheaths of severed axons as black particles on a light background—effectively isolating degenerating from healthy sheaths in tissue sections. Anatomists could produce discrete CNS lesions in experimental animals and after several weeks trace the course of neural pathways arising in the lesioned site. The method was severely limited, however, because unmyelinated or thinly myelinated axons, and terminal fields, were unlabeled, and there were many “false-positive” results from transecting fibers simply passing through the lesion (the “fiber-of-passage” problem common to many experimental methods).
By the 1950s selective silver impregnation and degeneration methods were combined by Walle J.H. Nauta and colleagues to stain unmyelinated axons and their terminal fields. The CNS was remapped at finer resolution with these methods, which still suffered from the fiber-of-passage problem (false-positive results) and were not nearly as sensitive (false-negative results) as the next generation of methods developed around 1970. Instead of relying on lesion-induced pathology the latter (current) are based on a combination of (1) physiological mechanisms (anterograde and retrograde intraaxonal marker transport) in healthy neurons and (2) histochemical detection of antibodies and complementary nucleic acid strands.
Also in the 1950s, the electron microscope opened a whole world of ultrastructure previously only guessed at. It provided the first glimpses of synapse structure (with a typical cleft only about 20 nm wide, far below the 1 mm resolution of light microscopes, and presynaptic vesicles), myelin sheath organization, and many cellular organelles. It also allowed biologists to examine in detail the biosynthetic apparatus residing within each cell.
These methods provide far more detail about CNS connectivity patterns or circuit organization than ever before in many species. As a result, comparative neuroanatomy has flourished and forms a solid structural foundation on which contemporary physiological and behavioral studies are based (Luo, Callaway, & Svoboda, 2008).
The PNS has Sensorimotor and Autonomic Divisions
Overall, the nervous system is divided into CNS (brain and spinal cord) and PNS (nerves, ganglia, and related plexuses). However, this CNS–PNS distinction is just a gross anatomical convenience that ignores circuit organization because nerves contain axons from neuronal cell bodies in both CNS and peripheral ganglia.
Functionally, the PNS has (1) a sensory ganglion component with accompanying dorsal and ventral roots and functionally mixed nerves, (2) the autonomic nervous system’s (ANS) motor ganglia and communicating roots, and (3) plexuses (literally “braids”). The sensory part has dorsal root ganglia sensory neurons with one extension (embryologically and phylogenetically an axon) entering the CNS through dorsal roots and the other extension (embryologically and phylogenetically a dendrite) traversing peripheral nerves to sites throughout the body. However, peripheral nerves also contain axons of skeletal motoneurons with cell bodies in spinal cord and brainstem; for spinal nerves the axon’s initial part traverses a ventral root. Thus, most peripheral nerves carry afferent (“sensory”) information toward the CNS and efferent (motor) information toward the body (Fig. 2.13). Sensory neurons carry afferent information from receptors in skin, skeletal muscles, tendons, joints, blood vessels, and deep viscera. The autonomic nervous system has a network of efferent pathways, ganglia, and nerve nets (plexuses) controlling gut peristalsis, glandular secretions, blood vessel diameter, and other visceral functions—and their output is modulated by both somatic and visceral afferents. Thus, a typical peripheral nerve carries a mixture of afferents and efferents innervating body wall and viscera.
Somatic afferents distribute near the body surface in a pattern reflecting the body wall’s segmental origins: each spinal nerve innervates a narrow mediolateral band of skin called a dermatome (Fig. 25.9), although adjacent nerves innervate overlapping territories (otherwise single nerve interruption would produce complete sensation loss in a band of skin, which is not the case). The segmental dermatome pattern is obvious in the torso where very little differential body wall growth occurs, whereas limb dermatomes are distorted because they form before the limbs grow out fully in the embryo.
Peripheral nerves often ramify and join with nerves from other segments to form nerve plexuses that serve as crossroads and distribution centers for the nerves, allowing axons to reorganize into complex nerve bundles innervating body structures. Brachial and lumbosacral plexuses near the origins of the upper and lower limbs, respectively, are the largest examples of these enigmatic structures that also provide great mechanical strength to nerves passing through the shoulders and hips, which may undergo extreme rotation and stretching. The ANS also has a bewildering variety of ganglionated plexuses in the abdomen and pelvis, where nerves converge and redistribute axons to their target organs and where individual neurons and/or clumps of autonomic neurons are found.
The ANS has structural and functional sympathetic and parasympathetic divisions (Chapter 34). The two divisions function in a kind of push-pull relationship with each other. One or the other is never completely on or off. Instead, there are degrees of sympathetic and parasympathetic tone. During sleep, certain involuntary functions like digestion are accelerated. Glands participating in digestion are activated parasympathetically and sympathetic tone is correspondingly decreased. In contrast, Walter B. Cannon noted almost a century ago that during the “fight or flight” reaction characterizing defensive behavior, sympathetic tone is markedly enhanced and parasympathetic tone is reduced sharply. Sympathetic outflow is vastly amplified and coordinated through a set of ganglia and the adrenal medulla, so that sympathetic function occurs relatively synchronously throughout the body. In contrast, the parasympathetic system is relatively finely tuned.
The Cerebrospinal Trunk Generates Cranial and Spinal Nerves
From a more systematic perspective on nervous system organization, the spinal cord and brainstem (together the cerebrospinal trunk) generate a continuous series of spinal and cranial nerves, respectively. The human spinal cord, roughly as thick as an adult’s little finger, is ultimately surrounded and protected by the vertebral column, whereas the cranial part of the skull protects the brain. In cross section, the spinal cord’s two basic types of nervous tissue are obvious: gray matter and white matter. Gray matter forms an H-shaped region surrounding the central canal (the ventricular system’s spinal segment) and consists mainly of neuronal cell bodies and neuropil. White matter surrounds gray matter in the spinal cord and consists mostly of axons collected into overlapping fiber bundles. Many axons have a myelin sheath, a uniquely vertebrate feature allowing rapid nerve impulse conduction (Chapter 4) and giving white matter its pale appearance.
The spinal cord looks segmented because bilateral pairs of dorsal and ventral roots emerge regularly along its length. These pairs form five sets: cervical (in the neck above the rib cage), thoracic (associated with the rib cage), lumbar (near the abdomen), sacral (near the pelvis), and coccygeal (associated with tail vertebrae). In humans there are typically 31 spinal nerve pairs (8 cervical, 12 thoracic, 5 lumbar, 5 sacral, and 1 coccygeal) that are named according to the intervertebral foramen they pass through. This enumeration varies between species.
Based on human brain macroscopic dissection, Samuel Thomas von Sömmerring in 1778 recognized a sequence of 12 cranial nerve pairs, and his basic classification scheme remains traditional for vertebrates in general, although it is problematic in terms of completeness (e.g., not including the nasal cavity’s terminal nerve) and nonconformance with contemporary fate maps of cranial nerve nucleus development (e.g., motoneurons for nerve VII are generated rostral to those for nerve VI). In any event, cranial nerves are more heterogeneous functionally than spinal nerves, and indeed most cranial nerve pairs have distinct compositions in terms of functional fiber types.
In humans (Fig. 2.19), seven cranial nerves transmit information about the so-called special senses associated with the head: olfaction (I, olfactory nerve—purely sensory, arising in nasal olfactory epithelium), vision (II, optic nerve from retina), hearing and balance (VIII, vestibulocochlear nerve from inner ear), and taste (V, VII, IX, and X; parts of the trigeminal, facial, glossopharyngeal, and vagus nerves, respectively). Nerves III (oculomotor), IV (trochlear), and VI (abducens) primarily control conjugate eye movements, although the third nerve also mediates autonomic control of the pupillary light reflex and lens accommodation. Major parts of the trigeminal (V) nerve carry sensory axons from the face (a rostral extension of the spinal somatosensory system) and motor axons innervating the muscles of mastication (chewing). The intermediofacial (VII) nerve controls the muscles of facial expression (facial nerve) and also innervates the salivary and lacrimal glands (intermediate nerve, VII’)—its role in emotional expression is obvious. The glossopharyngeal (IX) nerve innervates the tongue and pharynx and mediates the swallowing reflex. The vagus (“wandering,” X) nerve has an exceptionally complex and widespread innervation pattern, including laryngeal muscles producing speech, and the parasympathetic innervation of most thoracic and abdominal viscera. The accessory (XI) nerve innervates several muscles that stabilize the head and neck and the hypoglossal (XII) nerve innervates the tongue musculature.
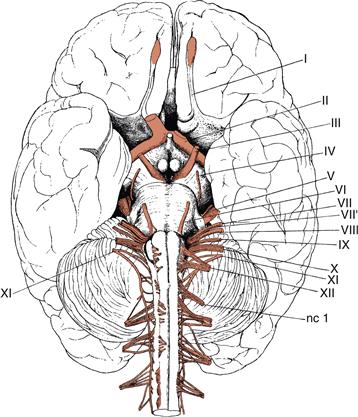
Figure 2.19 Origins of the cranial nerves viewed from the base of the adult human brain. The traditional numbering system dating back to the eighteenth century is indicated, but does not consider the terminal and accessory olfactory nerves. I, indicates the olfactory peduncle, with the olfactory bulb at the end shaded pink to indicate the termination site of the olfactory nerve; II, the optic tract leading from the optic chiasm to the thalamus and superior colliculus, with the stump of the right optic nerve indicated but the left optic nerve cut off at the chiasm; III, oculomotor nerve; IV, trochlear nerve, which courses around the side of the cerebral peduncle from the dorsal surface of the brainstem; V, trigeminal nerve; VI, abducens nerve; VII, facial nerve component of intermediofacial nerve; VII’, intermediate nerve component of intermediofacial nerve; VIII, vestibulocochlear nerve; IX, glossopharyngeal nerve; X, vagus nerve; XI, accessory nerve, with cranial and spinal roots; and XII, hypoglossal nerve; nc I, first cervical spinal nerve with the second through fourth also shown arising more caudally from the spinal cord. Cranial nerves exit through holes in the cranium whereas spinal nerves exit between the vertebrae (the first cranial nerve exits between the cranium and first vertebra).
Adapted from Henle (1871).
Cerebral Hemispheres and Cerebellum are Divided into Cortex and Nuclei
Macroscopically the mammalian cerebrospinal trunk has two great expansions—the cerebral hemispheres and cerebellum—and both have an outer laminated cortex surrounding deep nonlaminated nuclei. The most extraordinary growth of the mammalian brain occurs in the endbrain or cerebral hemispheres (Fig. 2.20), which develop more or less as mirror images of one another and are separated in the dorsal midline by a deep interhemispheric (longitudinal) fissure. In humans, the sulcal pattern is, however, asymmetric and unique in each person, and there are functional asymmetries as well; for example, the speech centers typically are lateralized (Chapter 49). Hemisphere volume is restricted by skull capacity, so as the hemispheres grow during embryogenesis they develop folds (gyri) separated by invaginations (sulci, and when deeper, fissures). This corrugation allows cerebral (and cerebellar) cortex to have a larger surface area. The extent and pattern of folding vary stereotypically with species, although like any trait there are quantitative differences between individuals of a particular species. Two major grooves, the central sulcus and lateral (Sylvian) fissure, are used as anatomical landmarks in the human cerebrum. The central sulcus extends roughly vertically along the hemisphere’s lateral surface where it approaches the horizontally oriented lateral fissure. Together they divide arbitrarily the lateral cerebral cortical surface into four lobes (frontal, parietal, occipital, and temporal), named for the overlying cranial bones. In addition, the insular lobe is folded completely inside the hemisphere, deep to the lateral fissure (actually about two-thirds of the folded cortical surface lies buried and unexposed to the outer hemisphere surface), and the limbic lobe forms the hemisphere’s medial border along the interhemispheric fissure.
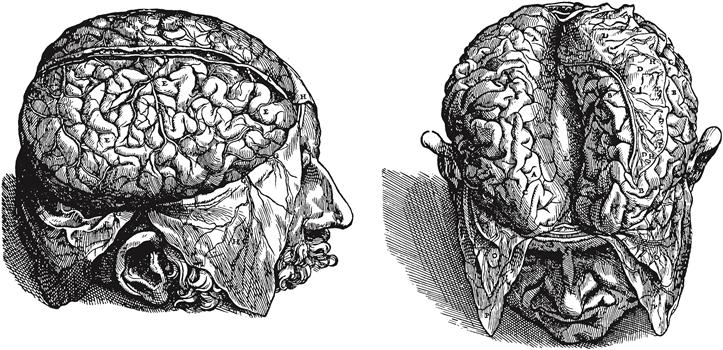
Figure 2.20 Surface features of the human cerebral cortex, which is thrown into gyri separated by sulci. In the drawing on the right, the right and left hemispheres have been pulled apart at the interhemispheric or longitudinal fissure to reveal a white matter tract, the corpus callosum (L), interconnecting the two hemispheres. The drawings are from perhaps the most important book in the history of medicine by Andreas Vesalius, Fabric of the Human Body, published in 1543. The drawings were probably executed by an artist from Titian’s studio. See Singer (1952).
These lobes are only crude guides to the cerebrum’s functional organization. Over the last 150 years, progressively better analysis has parceled the cortical mantle into a mosaic of roughly 50 to 100 areas with more or less distinct structural and functional characteristics. The most famous and enduring cortical regionalization maps were generated by Korbinian Brodmann a century ago (Fig. 2.21), although refinements and alternative interpretations abound. Nevertheless, cortical regionalization maps are fundamentally important guides for understanding CNS architecture. Just as one example, virtually the entire thalamus projects topographically on the cortical mantle, which in turn projects topographically on the entire cerebral nuclei (basal ganglia). Information from every sensory modality reaches the cerebral cortex and it in turn sends inputs to virtually the entire motor system.
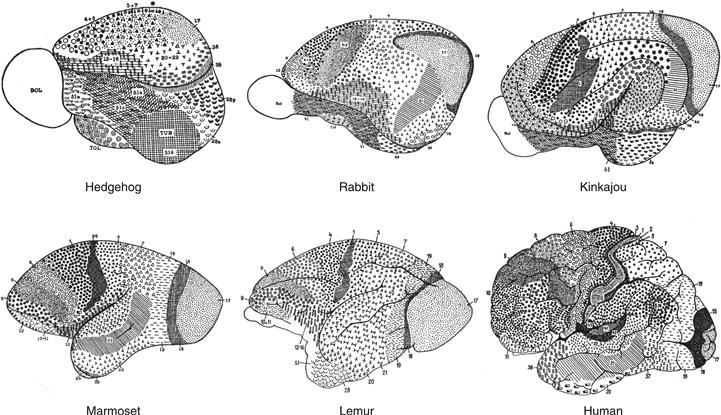
Figure 2.21 A similar cerebral cortical regionalization plan for mammals was proposed by Korbinian Brodmann in 1909. His cortical parceling was based on regional differences in how neuronal cell bodies tend to distribute in layers, an approach referred to as cytoarchitectonics. This figure summarizes his findings in six species, with different regions, or “areas,” as he called them, indicated with different symbols and numbers. He distinguished 47 areas in the human cerebral cortex and showed that generally similar patterns applied to all nine species he analyzed.
Most cerebral cortical areas directly modulate activity on the opposite (contralateral) side of the body through descending pathways that cross the midline to reach motor system parts in the contralateral CNS. Furthermore, axon bundles called commissures connect cerebral cortical areas of one hemisphere with the same or related areas of the opposite hemisphere—and different areas in the same hemisphere are interconnected through complex association pathways. Thus, commissural and association pathways allow comparison and integration of information between cortical areas within and between the cerebral hemispheres. Descending, commissural, and association connections tend to arise from different sets of pyramidal neurons, which helps explain the highly laminated organization of the cerebral cortex: descending connections arise mostly from the deep layers 5 and 6, commissural connections from layer 3, and association connections from layer 2.
Communication between hemispheres is eliminated by commissurotomy, the surgical division of all cerebral commissures (including the hippocampal and anterior commissures). This procedure sometimes is used to treat otherwise intractable epilepsy cases, preventing spread of severe epileptic activity from one hemisphere to the other. Incredibly, commissurotomy patients function very well most of the time, and behavioral studies on such “split brain” patients have yielded remarkable information about cerebral cortical organization (Gazzaniga, 2005). Axon bundles (tracts or pathways) connecting very different structures on the two sides of the CNS usually are called decussations to distinguish them from commissures.
The Nervous System is Protected by Membranous Coverings
The CNS is completely surrounded by three concentric connective tissue membranes: pia, arachnoid, and dura. The pia (“faithful”) is a very thin layer that adheres closely to the CNS’s surface, even where there are deep invaginations, as in the cerebral and cerebellar cortex. Then comes the arachnoid (“spidery”), which has a tenuous, web-like structure but is histologically similar to pia. Finally, the dura (“tough”) is a thick, inelastic covering apposed to the skull and vertebral canal’s inner surface (Fig. 2.20 H,O,P). Membranes covering the CNS are continuous with similar coverings of the PNS, where the terminology differs.
In certain CNS regions neural tissue is absent but meninges persist. Here, ependymal cells lining the ventricular system (the monolayer vestige of the embryonic neuroepithelial layer that ends up lining the adult ventricular system) fuse with the pia and arachnoid layers to form structures known as choroid plexus, which contains abundant blood vessels and serves as a component of the blood–brain barrier (the blood–CSF barrier; see Chapter 3). The choroid plexus produces CSF in the roof of the lateral, third, and fourth ventricles, and this fluid fills the brain ventricles, and perhaps at least part of the spinal central canal. Under positive hydrostatic pressure, CSF passes out of the brain’s interior through three foramina (holes) in the fourth ventricle’s roof, to fill the subarachnoid space between pia and arachnoid.
The Brain is Highly Vascular
The human brain consumes about 20% of the body’s oxygen supply at rest, even though it usually weighs only about a kilogram. Thus, the brain must continuously receive a voluminous blood supply, on the order of a liter per minute. Blood reaches the brain through two arterial roots—vertebral and internal carotid arteries—that anastomose in the circle of Willis, which essentially surrounds the base of the hypothalamus and pituitary gland’s stalk. The functional importance of this arterial circle cannot be overemphasized because afterward there is a drastic reduction in anastomoses between major arteries within brain tissue itself. As a result, blockage or rupture of an artery rapidly deprives the supplied brain region of oxygen, causing a stroke or brain attack.
After entering the skull through the foramen magnum with the spinal cord, the paired vertebral arteries fuse into a single basilar artery, generating the cerebellar arteries and the posterior cerebral arteries, which supply occipital cerebral regions. The internal carotid arteries divide to form the anterior and middle cerebral arteries; the former supplies each hemisphere’s medial surface (especially the limbic lobe), and the latter supplies the rest of the hemisphere (including the speech and somatic sensorimotor areas). By and large, the major arteries course along the cerebral surface and branches dive perpendicularly into the brain and proliferate into arterioles and capillaries.
A system of large venous sinuses collect blood from brain capillaries, and CSF from the subarachnoid space, and return them to the heart, mostly via the paired internal jugular veins. The major venous sinuses lie within the dura, whose inelasticity essentially holds the sinuses open. Blood flow through sinuses is slow and under low hydrostatic pressure. Thin-walled venous sinuses surrounded by the tough and immovable dura sets the stage for serious injury when the head is subjected to physical trauma. A well-known example is traumatic injury to the great cerebral vein (of Galen) in the midline that can occur when a boxer is struck in the head. The blow’s impact causes the brain to recoil in its CSF cushion, exerting a shearing force against the dura, which remains attached to the skull. This force may rupture the great cerebral vein, leading to serious hemorrhage of venous blood into the subdural space between dura and arachnoid.
Summary
This chapter reviews common approaches to the problem of understanding the nervous system’s fundamental structure and wiring diagram—the basic plan or architecture. One approach examines a series of increasingly complex animals from an evolutionary perspective to gain insight into basic organizing principles. It reveals trends toward centralization, cephalization, bilateral symmetry, and regionalization of the nervous system. It also suggests that basic molecular and cellular mechanisms of neuronal function, including electrical signal propagation and neurotransmitter release, have changed little since the appearance of the simplest nervous systems in hydra, jellyfish, and other cnidarians.
Another approach follows the vertebrate nervous system’s development from embryo to adult. At early developmental stages the CNS of all vertebrates has the same basic structure. A polarized, bilaterally symmetrical, regionalized neural plate of ectodermal origin invaginates to form a neural tube whose rostral half presents three swellings (forebrain, midbrain, and then hindbrain), followed by a caudal presumptive spinal cord. These four basic CNS divisions, arranged from rostral to caudal, go on to subdivide repeatedly until all laminated and nuclear neuron groups (regions) of the adult CNS are formed. A topographic, “geographic,” or regional account of the CNS emerges from this developmental approach.
How the CNS’s functional systems or circuitry are arranged into a unified whole is a tantalizing, deep, unsolved problem. The model discussed here equates behavior with motor output, which is driven by a combination of sensory, intrinsic behavioral state, and cognitive inputs—as well as by endogenous neuronal activity within each system. Future behavior is determined partly by sensory feedback related to the original behavior’s consequences. As this is written, the relationship between CNS macroregionalization (Figs. 2.14 and 2.15) and functional systems (Fig. 2.16) is not obvious. The correspondence between functional neural systems and gene expression networks is even more obscure, although promising results are beginning to emerge in the embryonic spinal cord and brainstem cranial nerve nuclei.
References
1. Alvarez-Bolado G, Swanson LW. Developmental brain maps: Structure of the embryonic rat brain Amsterdam: Elsevier; 1996.
2. Bota M, Swanson LW. Collating and curating neuroanatomical nomenclatures: principles and use of the Brain Architecture Knowledge Management System (BAMS). Front Neuroinform. 2010;4(3):1–16.
3. Brodmann, K. (1909). Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. Barth, Leipzig. Translated as Brodmann’s Localisation in the Cerebral Cortex by L. J. Garey. London: Gordon-Smith. 1994.
4. Brusca RC, Brusca GJ. Invertebrates Sunderland, MA: Sinauer Associates; 1990.
5. Cajal, S. Ramón y. (1909–1911). Histologie du système nerveux de l’homme et des vertébrés, in 2 vols., Maloine, Paris. Translated as Histology of the Nervous System of Man and Vertebrates by N. Swanson and L. W. Swanson. New York: Oxford University Press. 1995.
6. Cartmill M, Hylander WL, Shafland J. Human structure Cambridge, MA: Harvard University Press; 1987.
7. Gazzaniga MS. Forty-five years of split-brain research and still going strong. Nature Reviews Neuroscience. 2005;6:653–659.
8. Hamburger V. Anatomical and physiological basis of embryonic motility in birds and mammals. In: Gottlieb G, ed. New York: Academic Press; 1973;51076. Studies on the development of behavior and the nervous system. Vol. 1.
9. Henle J. Handbuch der Nervenlehre des Menschen Braunschweig: Vieweg; 1871.
10. Holland PW, Takahashi T. The evolution of homeobox genes: Implications for the study of brain development. Brain Research Bulletin. 2005;66:484–490.
11. Koizumi O. Developmental neurobiology of hydra, a model animal of cnidarians. Canadian Journal of Zoology. 2002;80:1678–1689.
12. Lentz TL. Primitive nervous systems New Haven: Yale University Press; 1968.
13. Luo L, Callaway EM, Svoboda K. Genetic dissection of neural circuits. Neuron. 2008;57:634–660.
14. McConnell CH. Development of the ectodermal nerve net in the buds of Hydra. The Quarterly Journal of Microscopical Science. 1932;75:495–509.
15. Nieuwenhuys R, Voogd J, van Huijzen C. The human central nervous system: A synopsis and atlas 3rd ed. Berlin: Springer-Verlag; 1988.
16. Parker GH. The elementary nervous system Philadelphia: Lippincott; 1919.
17. Peters A, Palay SL, Webster H deF. The fine structure of the nervous system: Neurons and their supporting cells 3rd ed. New York: Oxford University Press; 1991.
18. Sherrington CS. The integrative action of the nervous system New York: Scribner’s; 1906; (Reprinted, Yale University Press, New Haven, 1947).
19. Sidman RL, Angevine Jr JB, Taber Pierce E. Atlas of the mouse brain and spinal cord Cambridge, MA: Harvard University Press; 1971.
20. Singer C. Vesalius on the Human Brain: Introduction Oxford University Press: Oxford; 1952; Translation of the Text, Translation of Descriptions of Figures, Notes to the Translations, Figures.
21. Sporns O, Tonono G, Kötter R. The human connectome: a structural description of the human brain. PLoS Computational Biology. 2005;1(4):0245–0251.
22. Swanson LW. Brain maps: structure of the rat brain Amsterdam: Elsevier; 1992.
23. Swanson LW. What is the brain?. Trends in Neuroscience. 2000a;23:519–527.
24. Swanson LW. A history of neuroanatomical mapping. In: Toga AW, Mazziotta JC, eds. Brain mapping: The applications. San Diego: Academic Press; 2000b;77–109.
25. Swanson LW. Brain architecture: understanding the basic plan 2nd ed. Oxford: Oxford University Press; 2012.
26. Von Neumann J. The computer and the brain New Haven: Yale University Press; 1958.
27. Wiener N. Cybernetics, or control and communication in the animal and machine New York: Wiley; 1948.
28. Williams PL, ed. Gray’s anatomy. New York: Churchill Livingstone; 1995.
Suggested Readings
1. Bergquist H, Källén B. Notes on the early histogenesis and morphogenesis of the central nervous system in vertebrates. The Journal of Comparative Neurology. 1954;100:627–659.
2. Björklund A, Hökfelt T. Handbook of chemical neuroanatomy Amsterdam: Elsevier; 1983–present.
3. Bohland JW, Wu C, Barbas H, et al. A proposal for a coordinated effort for the determination of a brainwide neuroanatomical connectivity in model organisms at a mesoscopic scale. PLoS Computational Biology. 2009;5(3):1–9.
4. Descartes R. Treatise on Man Harvard University Press: Cambridge; 1972; French text with translation by T. S. Steele.
5. Herrick CJ. The brain of the tiger salamander Chicago: University of Chicago Press; 1948.
6. Jones AR, Overly CC, Sunkin SM. The Allen Brain Atlas: 5 years and beyond. Nature Reviews Neuroscience. 2009;10:1–8.
7. Kingsbury BF. The fundamental plan of the vertebrate brain. The Journal of Comparative Neurology. 1922;34:461–491.
8. Lorenz K. Behind the mirror Orlando, FL: Harcourt Brace Jovanovich; 1978.
9. Russell ES. Form and function: A contribution to the history of animal morphology London: John Murray; 1916.
10. Swanson LW, Bota M. Foundational model of nervous system structural connectivity with a schema for wiring diagrams, connectome, and basic plan architecture. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:20610–20617.
11. Tinbergen N. The study of instinct London: Oxford University Press; 1951.